Ground-Water Quality Classification and Recommended Septic Tank Soil- Absorption-System Density Maps, Cache Valley, Cache County, Utah
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

THE WHY and Wherefore Or POOR RADIO RECEPTION
Modern radios are pack ed w ith features and refin ements that add immeasurably to radio enjoyment. Yet , no amount of radio improve - ments can increase th is enjoyment 'unless these improvements are u sed-and used properly . Ev en older radios are seldom operated to bring out the fine performance which they are WITH capable of giving . So , in justice to yourself and ~nninqhom the fi ne radio programs now being transmitted , ask yoursel f this questi on: "A m I getting as much enjoyment from my r ad io as possible?" Proper radio o per atio n re solves itself into a RADIO TUBES matter of proper tunin g. Yes , it's as simple as that . But you would be su rprised how few Hour aft er hour .. da y a nd night ... all ye ar people really know ho w t o tune a radio . In lon g . .. th e air is fill ed with star s who enter- Figure 1, the dial pointer is shown in the tain you. News broad casts ke ep you abrea st of middle of a shaded area . A certain station can be heard when the pointer covers any part of a swiftl y moving world . .. sport scast s brin g this shaded area , but it can only be heard you the tingling thrill of competition afield. enjo yably- clearl y and without distortion- Yet none of the se broadca sts can give you when the pointer is at dead center , midway between the point where the program first full sati sfaction unle ss you hear th em properl y. -

2004 Utah State Football
UTAH STATE FOOTBALL QUICK FACTS 2004 UTAH STATE FOOTBALL University Quick Facts Team Quick Facts Location: Logan, Utah 2003 Overall Record: 3-9 Founded: 1888 Sun Belt Conf. Record: 3-4 (tie 4th) Enrollment: 21,490 Basic Offense: One Back President: Dr. Kermit L. Hall (Akron, 1966) Basic Defense: 3-4 Director of Athletics: Randy Spetman (Air Force, 1976) Lettermen Returning: 43 (18 Off., 23 Def., 2 Spec.) Conference: Sun Belt Lettermen Lost: 24 (13 Off., 11 Def., 0 Spec.) Nickname: Aggies Returning Starters (2003 starts) Colors: Navy Blue and White Offense (4) Stadium: Romney Stadium (30,257) LT - Donald Penn (12) Turf: Sprinturf (installed summer of 2004) RT - Elliott Tupea (10) will play RG in 2004 WR - Raymond Hicks (7) Coaching Quick Facts QB - Travis Cox (12) Head Coach: Mick Dennehy (Montana, 1973) Defense (6) Record at USU: 16-29 (four years) NG - Ronald Tupea (12) Overall Record: 65-54 (11 years) RT - John Chick (9) will play LB in 2004 Linebackers -- Lance Anderson (Idaho State, 1996), 1st Year MLB - Robert Watts (12) Spec. Teams/Safeties -- Jeff Choate (W. Montana, 1993), 2nd Year WLB - Nate Fredrick (9) Off. Coord./QB -- Bob Cole (Widener, 1982), 5th Year LC - Cornelius Lamb (7) Offensive Line -- Jeff Hoover (UC Davis, 1991), 5th Year FS - Terrance Washington (8) Def. Coord. -- David Kotulski (N.M. State, 1975), 2nd Year Starters Lost (2003 starts) Tight Ends -- Mike Lynch (Montana, 1999), 3rd Year Offense (7) Defensive Line -- Tom McMahon (Carroll, 1992), 7th Year LG - Greg Vandermade (12) Secondary -- John Rushing (Wash. State, 1995), 2nd Year OC - Aric Galliano (12) Wide Receivers/Asst. -

CACHE COUNTY COUNCIL MEETING March 11, 2008
CACHE COUNTY COUNCIL MEETING March 11, 2008 The Cache County Council convened in a regular session on March 11, 2008 in the Cache County Council Chamber at 199 North Main, Logan, Utah. ATTENDANCE: Chairman: John Hansen, absent. Vice Chairman: H. Craig Petersen Council Members: Darrel Gibbons, Kathy Robison, Cory Yeates & Gordon Zilles. Brian Chambers, absent. County Executive: M. Lynn Lemon County Clerk: Jill N. Zollinger County Attorney: James Swink N. George Daines, absent. The following individuals were also in attendance: James Astle, Davis Coppin, Garth Day, Vern Elwood, Jeff Gilbert, Dirk Henningsen, Sharon L. Hoth, Dan Hunsaker, Scott Hyde, Tawni Hyde, Chad Jensen, Wayne Lewis, David Mann, Terry Mann, Sheriff Lynn Nelson, Brandon Nham, David Nielsen, Pat Parker, Dave Petersen, Russ Piggott, Josh Runhaar, Jim Smith, Larry Soule, Jay Stocking, Zan Summers, Kai Torrens, Mayor Cary Watkins, Aaron Wiser, Carter Young, Media: Charles Geraci (Herald Journal), Arrin Brunson, (Salt Lake Tribune), Jennie Christensen (KVNU). OPENING REMARKS AND PLEDGE OF ALLEGIANCE Council member Gibbons gave the opening remarks and led those present in the Pledge of Allegiance. REVIEW AND APPROVAL OF AGENDA ACTION: Motion by Council member Yeates to approve the agenda as written. Robison seconded the motion. The vote was unanimous, 5-0. Chambers & Hansen absent. REVIEW AND APPROVAL OF MINUTES ACTION: Motion by Council member Yeates to approve the minutes of the February 26, 2008 Council meeting as written. Gibbons seconded the motion. The vote was unanimous, 5-0. Chambers & Hansen absent. REPORT OF THE COUNTY EXECUTIVE: M. LYNN LEMON APPOINTMENTS: There were no appointments WARRANTS: There were no warrants. -

The Mountain View Inn!
Welcome to the Mountain View Inn! On behalf of the 75th ABW, the 75th Force Support Squadron, and the Mountain View Inn Staff, welcome to Hill Air Force Base, Headquarters for the Ogden Air Logistics Center. We are honored to have you as our guest and sincerely hope your visit to Hill Air Force Base and the Layton/Salt Lake City area is an exceptional one. Please take a few minutes to review the contents of this book to discover the outstanding services available at both Hill Air Force Base and the surrounding area. If there is anything we can do to make your visit more comfortable, or if you have any suggestions on how we can improve our service, please fill out a Customer Comment Card located in your room or at our Guest Reception Desk. The Mountain View Inn is a recipient of both the prestigious Air Force Material Command Gold Key Award and the Air Force Innkeeper Award. We are truly dedicated to providing quality service to you, our valued guest, and are available 24 hours a day to assist you and make your stay a memorable one. The Mountain View Inn team of professionals wishes you a pleasant stay and a safe journey. We look forward to serving you and hope to see you again in the future! Melissa L. Edwards Lodging Manager 801-777-1844 EXT 2560 Welcome Valued Guest! We have provided you with a few complimentary items to get you through your first night’s stay. Feel free to ask any Lodging team member if you need any of these items replenished. -

Department of the Interior Miscellaneous Field Studies United States Geological Survey Map Mf-1566-A Pamphlet
DEPARTMENT OF THE INTERIOR MISCELLANEOUS FIELD STUDIES UNITED STATES GEOLOGICAL SURVEY MAP MF-1566-A PAMPHLET MINERAL RESOURCE POTENTIAL OF MOUNT NAOMI ROADLESS AREA, CACHE COUNTY, UTAH, AND FRANKLIN COUNTY, IDAHO By James H. Dover, U.S. Geological Survey and Philip R. Bigsby, U.S. Bureau of Mines STUDIES RELATED TO WILDERNESS Under the provisions of the Wilderness Act (Public Law 88-577, September 3, 1964) and related acts, the U.S. Geological Survey and the U.S. Bureau of Mines have been conducting mineral surveys of wilderness and primitive areas. Areas officially designated as "wilderness," "wild," or "canoe" when the act was passed were incorporated into the National Wilderness Preservation System, and some of them are presently being studied. The act provided that areas under consideration for wilderness designation should be studied for suitability for incorporation into the Wilderness System. The mineral surveys constitute one aspect of the suitability studies. The act directs that the results of such surveys are to be made available to the public and be submitted to the President and the Congress. This report discusses the results of a mineral survey of the Mount Naomi Roadless Area (04758), Wasatch and Caribou National Forests, Cache County, Utah, and Franklin County, Idaho. Mount Naomi Roadless Area was classified as a further planning area during the Second Roadless Area Review and Evaluation (RARE n)i by the U.S. Forest Service, January 1979. MINERAL RESOURCE POTENTIAL SUMMARY STATEMENT Geological, geophysical, and geochemical investigations have been conducted to assess the mineral resource potential of the Mount Naomi Roadless Area (hereafter referred to as "study area"), northeastern Utah and southeastern Idaho. -

Wasatch-Cache National Forest Plan
; The Stilt Volume 30, Is* October 2001 I Newsletter of the Bridgerland Audubon Society Wasatch-Cache National Forest Plan comments Comments on the proposed areas for winter non-motorized everywhere are being pushed Forest Plan and DEIS for the use and adopt an implementa• into smaller and smaller Wasatch-Cache National Forest tion plan for the eventuality that habitats. are due November 1. This latest they won't have adequate round of the Plan is much resources to monitor and patrol In 1999, the Forest Service improved from the preliminary to stop damaging uses. Motor• released the first pass at a new versions put forth in 1999 and ized recreationists have sent forest plan, the Preliminary 2000, but it still needs improve• hundreds of postcards, but Assessment of the Management ments — improvements that altogether, they're not worth one Situation, or PAMS. (Yes, this won't happen unless our voices well written letter. And it doesn't whole process overflows with are heard. The Forest Service is have to be Shakespearean jargon and acronyms, but it IS under great pressure from poetry, either. They want simple possible for the layperson to motorized recreationists and statements—dot points—that make sense of it.) BAS submit• livestock grazers to weaken spell out what you've seen, what ted comments summarized in environmental protections, but you want, and why it's important. the December 1999 issue of the our letters can offset many of Read on, then write that letter Stilt. In 2000, the USFS re• these pro-development TONIGHT! leased some draft alternatives, "postcard" responses. -

Structural and Lithological Influences on the Tony Grove Alpine Karst
Utah State University DigitalCommons@USU All Graduate Theses and Dissertations Graduate Studies 5-2016 Structural and Lithological Influences on the onyT Grove Alpine Karst System, Bear River Range, North-Central Utah Kirsten Bahr Utah State University Follow this and additional works at: https://digitalcommons.usu.edu/etd Part of the Geology Commons Recommended Citation Bahr, Kirsten, "Structural and Lithological Influences on the onyT Grove Alpine Karst System, Bear River Range, North-Central Utah" (2016). All Graduate Theses and Dissertations. 5015. https://digitalcommons.usu.edu/etd/5015 This Thesis is brought to you for free and open access by the Graduate Studies at DigitalCommons@USU. It has been accepted for inclusion in All Graduate Theses and Dissertations by an authorized administrator of DigitalCommons@USU. For more information, please contact [email protected]. STRUCTURAL AND LITHOLOGICAL INFLUENCES ON THE TONY GROVE ALPINE KARST SYSTEM, BEAR RIVER RANGE, NORTH-CENTRAL UTAH by Kirsten Bahr A thesis submitted in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE in Geology Approved: ______________________________ ______________________________ W. David Liddell, Ph.D. Robert Q. Oaks, Jr, Ph.D. Major Professor Committee Member ______________________________ ______________________________ Thomas E. Lachmar, Ph.D. Mark R. McLellan, Ph.D. Committee Member Vice President for Research and Dean of the School of Graduate Studies UTAH STATE UNIVERSITY Logan, Utah 2016 ii Copyright © Kirsten Bahr 2016 All Rights Reserved iii ABSTRACT Structural and Lithological Influences on the Tony Grove Alpine Karst System, Bear River Range, North-Central Utah by Kirsten Bahr, Master of Science Utah State University, 2016 Major Professor: Dr. W. -
U. S. Radio Stations As of June 30, 1922 the Following List of U. S. Radio
U. S. Radio Stations as of June 30, 1922 The following list of U. S. radio stations was taken from the official Department of Commerce publication of June, 1922. Stations generally operated on 360 meters (833 kHz) at this time. Thanks to Barry Mishkind for supplying the original document. Call City State Licensee KDKA East Pittsburgh PA Westinghouse Electric & Manufacturing Co. KDN San Francisco CA Leo J. Meyberg Co. KDPT San Diego CA Southern Electrical Co. KDYL Salt Lake City UT Telegram Publishing Co. KDYM San Diego CA Savoy Theater KDYN Redwood City CA Great Western Radio Corp. KDYO San Diego CA Carlson & Simpson KDYQ Portland OR Oregon Institute of Technology KDYR Pasadena CA Pasadena Star-News Publishing Co. KDYS Great Falls MT The Tribune KDYU Klamath Falls OR Herald Publishing Co. KDYV Salt Lake City UT Cope & Cornwell Co. KDYW Phoenix AZ Smith Hughes & Co. KDYX Honolulu HI Star Bulletin KDYY Denver CO Rocky Mountain Radio Corp. KDZA Tucson AZ Arizona Daily Star KDZB Bakersfield CA Frank E. Siefert KDZD Los Angeles CA W. R. Mitchell KDZE Seattle WA The Rhodes Co. KDZF Los Angeles CA Automobile Club of Southern California KDZG San Francisco CA Cyrus Peirce & Co. KDZH Fresno CA Fresno Evening Herald KDZI Wenatchee WA Electric Supply Co. KDZJ Eugene OR Excelsior Radio Co. KDZK Reno NV Nevada Machinery & Electric Co. KDZL Ogden UT Rocky Mountain Radio Corp. KDZM Centralia WA E. A. Hollingworth KDZP Los Angeles CA Newbery Electric Corp. KDZQ Denver CO Motor Generator Co. KDZR Bellingham WA Bellingham Publishing Co. KDZW San Francisco CA Claude W. -

Next Issue Stopdate and Address for Loggings and Gossips: 23.9.2008 Nro 14
23.9.2008 Nro 14 965 Next issue stopdate and address for loggings and gossips: MONDAY, 13.10.2008 to: Jari Lehtinen, Saimaankatu 7 C 51, 15140 LAHTI web: http://clusive.sdxl.org email: [email protected] Editor-in-Chief: Jari Lehtinen (JLN)........ …[email protected] ……...….…….…. 03 - 7830 598 Euronews: Jarmo Patala (JP)................ [email protected] .......... 0400 – 610301 Afronews: Jari Korhonen (JJK)……… Asian-Oceanian News: Jari Savolainen (JSA)……. [email protected] ………………….05 - 3631 791 North-American Newswatch:Tapio Kalmi (TAK).......…[email protected] ................050 – 521 6027 Ultimas Noticias: Jari Lehtinen (JLN)………[email protected] ………………….03 – 7830 598 Loggings & Hot News: Jari Lehtinen (JLN)........…. [email protected]...……….…....... 03 - 7830 598 Brittein saaret 1215 20.9. 0400- G: Virgin Radio. Mukavaa aamukaffiohjelmaa ja kuuntelijakilpailuita auringonnousun aikoihin. TTM (Toimittaja huomauttaa: sain lahjoituksena Sansuin viritinvahvistimen vuosikertaa 1982-1984. Ällistys oli suuri. Sillä värkillä kuuluu kivitalossa keskustassa Virgin Radio loistavasti, monin verroin paremmin kuin K-PO WR2100:lla – tai muulla vastaavalla matkaradiolla, veikkaisin. /jln) Iberia 1116 19.9. -2100 E: SER R Pontevedra. Miniwhip on hintaansa ja kokoonsa nähden mainio antenni mutta sitä pitää käyttää häiriöttömissä olosuhteissa ja sijoittaa niin korkealle kuin pystyy. VJR/SS 1161 19.9. 2300- E: Euskadi Irratia, San Sebastian. VJR/SS 1224 28.8. 0300- E: Herri Irratia, San Sebastian. Tukevasti peittäen COPEt. VJR/N Afrikka 1053.1 18.9. 2159- LBY: LJB Tripoli. Kuuntelin Saariselällä 17.-20.9 patikointireissujen lomassa. Varusteena SDR-IQ ja lomakylän lipputankoon hissattu Miniwhip. Yöaikaan MW-bandi oli suhteellisen häiriötön ja mielenkiintoisia havaintoja löytyi. Esim. 0300 UTC COPE kepitti Virgin Radion 1215 kHz. -

Intermountain Trails
Intermountain Trails USDA Forest Service—Intermountain Region August 2014 Volume 3, Issue 6 Forest In Focus: Uinta-Wasatch-Cache National Forest Forest Supervisor—Dave Whittekiend In this issue: The Uinta-Wasatch-Cache National Forest Forest in Focus 1 was recently recognized as the National Volunteer Program Unit of the Year. The Forest Supervisor Message forest hosted over 12,000 volunteers for a total of 83,196 hours of work. This volunteer Trail Stories workforce accomplishes a wide variety of work on the ground, from naturalist walks to Trail Stories 2 Notch Mountain trail construction and maintenance. Without Capitol this dedicated unpaid workforce, many of the Improvement Uinta-Wasatch-Cache Forest Supervisor, services our public expects and enjoys would Project Dave Whittekiend not exist. This incredible volunteer program is made possible by the dedicated and passionate National Public 3 Lands Day employees of the Uinta-Wasatch-Cache. Our volunteer coordinators are continuously making connections with people and projects. It could be linking Safety Tip dedicated hunters to a guzzler installation or showing a prospective Eagle Scout a Mount Naomi 4 trailhead in need of improvement. The UWC volunteer coordinators and the rest Wilderness of the employees on the forest are always willing to figure out how to include volunteers in managing this incredible resource we call the uinta-Wasatch-Cache National Forest. Uinta‐Wasatch‐Cache Wellsville 5 NaƟonal Forest Mountain 857 West South Jordan Parkway Wilderness South Jordan, Utah hƩp://www.fs.usda.gov/uwcnf/ High Uintas 6 Wilderness TRAIL STORIES Notch Mountain Capitol Improvement Project-see page 2 1 Notch Mountain Capitol Improvement Project Trail Stories Through the use of a State of Utah, Recreation Trails Program grant, the Heber-Kamas Ranger District has made significant improvements to the Notch Mountain Trail in the first year of the multi-year grant. -

Beaver Cedar City Delta
Beaver Cedar City Delta KBBD Silent [Z] KSUB Talk/Soft AC [TK&/SA] KNAK Country [CW] 90.1 10w 72ft 590 5000/1 OOOw DA-N 540 1000/30W ND 6s 2s Beaver County School District Southern Utah Bcstg Co. +Douglas Barton 801- Sister KSSD 801-864-5111 fax 801-835-2250 801-586-5900 fax 801-586-0437 Box 636, 84624 Blanding Box 819, 84721 GM Doug Barton GM Gerald Johnson KFMD Country [CW] KBRE Oldies [OL&] KUTA Talk/Country [TK/CW] 95.7 100000W 7ft 9n 3s 9c 3c 3t 790 1000/w ND-D 7s 940 10000/w ND-D 3s 2t North Star Media Corp. Sister KSGI also owns KSGI-FM Mueller Broadcasting, Inc. Kolob Broadcast Radio Enterprises 801 -678-2261 fax 801-678-2262 Sister KBRE-FM 801 -864-4500 fax 801 -628-6636 Hwy. 191 N, 84511 801-586-5273 fax 801-586-0458 Box 696, 84624 426 E. Topaz Blvd., 84624 GM Phil Mueller Box 858, 84720 450 W. 400 S, 84720 GM Neil Farnsworth GM Art Challis Bountiful KGSU-FM Religion [RL*] Ephraim 91.1 10OOOw -462ft KUTQ CHR [CH] Southern Utah State College KAGJ [cp-new] 99.5 40000W 2953ft 8s9c1c9t0t 801-586-7975 fax 801-586-7934 89.5 100w -321ft Bountiful Broadcasting, Inc. 351 W. Center, 84720 Snow College Sister KTKK LMA controls KZHT GM Lee Byers 801- 801 -264-8250 fax 801 -264-8978 KSSD Country [CW&] 3595 S. 1300 W, Salt Lake City 84119 92.5 41600W 1690ft Heber City GM/PD Gary Waldron SM Bruce Corrigan Southern Utah Bcstg Co. -
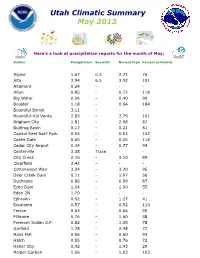
Utah Climatic Summary May 2013
Utah Climatic Summary May 2013 Here’s a look at precipitation reports for the month of May: Station Precipitation Snowfall Normal Pcpn Percent of Normal Alpine 1.67 0.3 2.21 76 Alta 3.94 6.5 3.92 101 Altamont 0.24 - - - Alton 0.85 - 0.72 118 Big Water 0.36 - 0.40 90 Boulder 1.18 - 0.64 184 Bountiful Bench 3.11 - - - Bountiful-Val Verda 2.83 - 2.79 101 Brigham City 1.81 - 2.08 87 Bullfrog Basin 0.17 - 0.21 81 Capitol Reef Nat’l Park 0.54 - 0.53 102 Castle Dale 0.65 - 0.55 118 Cedar City Airport 0.34 - 0.77 44 Centerville 3.38 Trace - - City Creek 2.76 - 3.10 89 Clearfield 2.42 - - - Cottonwood Weir 3.04 - 3.20 95 Deer Creek Dam 0.71 - 1.97 36 Duchesne 0.86 - 0.99 87 Echo Dam 1.04 - 1.90 55 Eden 2N 1.70 - - - Ephraim 0.52 - 1.27 41 Escalante 0.57 - 0.52 110 Ferron 0.63 - 0.66 95 Fillmore 0.76 - 1.60 48 Fremont Indian S.P. 0.82 - 1.05 78 Garfield 1.78 - 2.48 72 Hans Flat 0.56 - 0.60 93 Hatch 0.55 - 0.76 72 Heber City 0.42 - 1.43 29 Helper-Carbon 1.06 - 1.03 103 Station Precipitation Snowfall Normal Pcpn Percent of Normal Huntsville 1.57 - 2.10 75 Kanab 0.22 - 0.55 40 Kodachrome Basin S.P. 0.45 - 0.57 79 Laketown 1.60 - 1.65 97 Layton 2.24 - - - Levan 0.69 - 1.61 43 Loa 0.84 - 0.61 138 Logan KVNU 1.33 - 2.03 66 Manti 1.09 - 1.48 74 Milford 0.76 - 0.76 100 Mountain Dell 2.54 - 3.00 85 Neola 0.25 - 1.01 25 Nephi 1.16 Trace 1.62 72 Ogden NE Bench 2.39 Trace - - Orderville 0.13 0.72 18 Pineview Dam 1.83 Trace 3.32 55 Pleasant Grove 1.26 - 2.00 63 Provo BYU 0.89 - 2.58 34 Randolph 0.71 - 1.75 41 Richfield 0.65 - 1.05 62 Salt Lake Triad Center