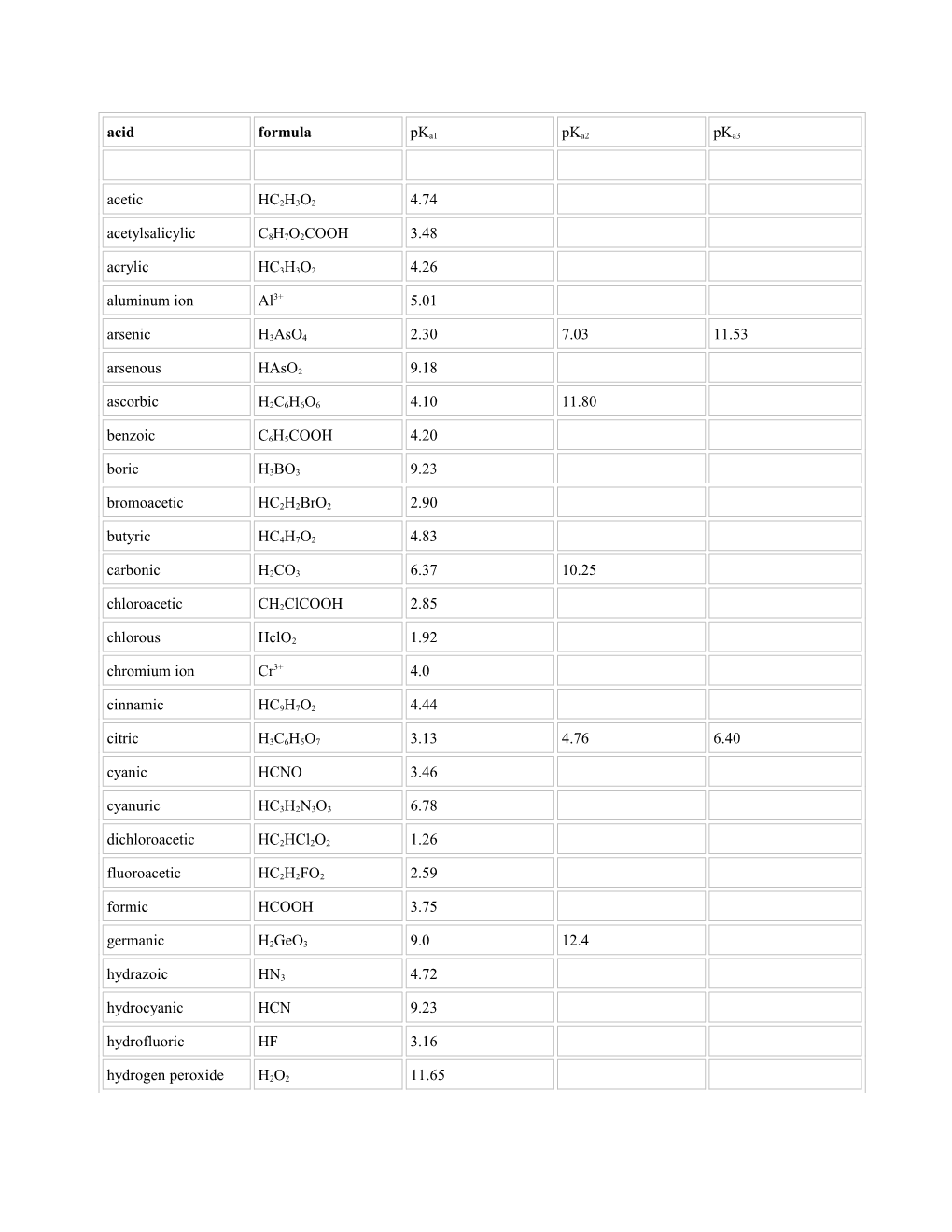
acid formula pKa1 pKa2 pKa3
acetic HC2H3O2 4.74 acetylsalicylic C8H7O2COOH 3.48 acrylic HC3H3O2 4.26 aluminum ion Al3+ 5.01 arsenic H3AsO4 2.30 7.03 11.53 arsenous HAsO2 9.18 ascorbic H2C6H6O6 4.10 11.80 benzoic C6H5COOH 4.20 boric H3BO3 9.23 bromoacetic HC2H2BrO2 2.90 butyric HC4H7O2 4.83 carbonic H2CO3 6.37 10.25 chloroacetic CH2ClCOOH 2.85 chlorous HclO2 1.92 chromium ion Cr3+ 4.0 cinnamic HC9H7O2 4.44 citric H3C6H5O7 3.13 4.76 6.40 cyanic HCNO 3.46 cyanuric HC3H2N3O3 6.78 dichloroacetic HC2HCl2O2 1.26 fluoroacetic HC2H2FO2 2.59 formic HCOOH 3.75 germanic H2GeO3 9.0 12.4 hydrazoic HN3 4.72 hydrocyanic HCN 9.23 hydrofluoric HF 3.16 hydrogen peroxide H2O2 11.65 hydroselenic H2Se 3.89 11.0 hydrosulfuric H2S 6.88 14.15 hydrotelluric H2Te 2.64 10.80 hypobromous HBrO 9.24 hypochlorous HClO 7.55 hypoiodous HIO 10.64 hyponitrous H2N2O2 7.05 11.4 hypophosphorous H3PO2 1.23 iodic HIO3 0.80 iodoacetic HC2H2IO2 3.18 iron(II)ion Fe2+ 6.74 iron(III)ion Fe3+ 2.83 lactic HC3H5O3 3.08 maleic HOOCCH:CHCOOH 1.84 6.07 malonic H2C3H2O4 2.82 5.70 nitrous HNO2 3.14 oxalic H2C2O4 1.23 4.19 phenol HOC6H5 10.00 phosphoric H3PO4 2.12 7.21 12.67 phosphorous H3PO3 1.80 6.15 phthalic H2C8H4O4 2.92 5.41 propionic HC3H5O2 4.87 salicylic HC7H5O3 1.96
-3 selenic H2SeO4 1.66 selenous H2SeO3 2.64 8.27 succinic H2C4H4O4 4.21 5.64 sulfuric H2SO4 none 1.92 sulfurous H2SO3 1.89 7.21 thiophenol HSC6H5 6.49 trichloroacetic HC2Cl3O2 0.52 zinc ion Zn2+ 8.96
base formula pKb1 pKb2
ammonia NH3 4.76 aniline C6H5NH2 9.37 codeine C18H21O3N 6.05 diethylamine (C2H5)2NH 4.51 dimethylamine (CH3)2NH 3.23 ethylamine C2H5NH2 3.36 hydrazine N2H4 5.77 15.05 hydroxylamine HONH2 9.04 methylamine CH3NH2 3.38 morphine C17H19O3N 6.13 piperidine C5H11N 2.88 pyridine C5H5N 8.70 quinoline C9H7N 9.20 triethanolamine C6H15O3N 6.24 triethylamine (C2H5)3N 3.28 trimethylamine (CH3)3N 4.20
Acid Formula Ka1 Ka2 Ka3 -5 Acetic CH3COOH 1.75x10 + -10 Ammonium Ion NH4 5.70x10 + -5 Anilinium Ion C6H5NH3 2.51x10 -3 -7 -12 Arsenic H3AsO4 5.8x10 1.1x10 3.2x10 -10 Arsenous H3AsO3 5.1x10 -5 Benzoic C6H5COOH 6.28x10 -10 Boric H3BO3 5.81x10 -5 1-Butanoic CH3CH2CH2COOH 1.52x10 -7 -11 Carbonic H2CO3 4.45x10 4.69x10 -3 Chloroacetic ClCH2COOH 1.36x10 -4 -5 -7 Citric HOOC(OH)C(CH2COOH)2 7.45x10 1.73x10 4.02x10 Formic HCOOH 1.80x10-4 Fumaric trans-HOOCCH:CHCOOH 8.85x10-4 3.21x10-5 -4 Glycolic HOCH2COOH 1.47x10 + -8 Hydrazinium Ion H2NNH3 1.05x10 -5 Hydrazoic HN3 2.2x10 Hydrogen Cyanide HCN 6.2x10-10 Hydrofluoric HF 6.8x10-4 -12 Hydrogen Peroxide H2O2 2.2x10 -8 -14 Hydrogen Sulfide H2S 9.6x10 1.3x10 + -6 Hydroxyl Ammonium Ion HONH3 1.10x10 Hydrochloric HCl Strong Hypochlorous HOCl 3.0x10-8 -1 Iodic HIO3 1.7x10 -4 Lactic CH3CHOHCOOH 1.38x10 Maleic cis-HOOCCH:CHCOOH 1.3x10-2 5.9x10-7 -4 -6 Malic HOOCCHOHCH2COOH 3.48x10 8.00x10 -3 -6 Malonic HOOCCH2COOH 1.42x10 2.01x10 -4 Mandelic C6H5CHOHCOOH 4.0x10 + -11 Methyl Ammonium Ion CH3NH3 2.3x10
Nitric HNO3 Strong -4 Nitrous HNO2 7.1x10 Oxalic HOOCCOOH 5.60x10-2 5.42x10-5
Perchloric HClO4 Strong -2 -9 Periodic H5IO6 2x10 5x10 -10 Phenol C6H5OH 1.00x10 -3 -8 -13 Phosphoric H3PO4 7.11x10 6.32x10 4.5x10 -2 -7 Phosphorous H3PO3 3x10 1.62x10 -3 -6 o-Phthalic C6H4(COOH)2 1.12x10 3.91x10 -1 Picric (NO2)3C6H2OH 4.3x10 + -12 Piperidinium C5H11NH 7.50x10 -5 Propanoic CH3CH2COOH 1.34x10 + -6 Pyridinium C5H5NH 5.90x10 -3 Salicylic C6H4(OH)COOH 1.06x10 -1 Sulfamic H2NSO3H 1.03x10 -5 -6 Succinic HOOCCH2CH2COOH 6.21x10 2.31x10 -2 Sulfuric H2SO4 Strong 1.02x10 -2 -8 Sulfurous H2SO3 1.23x10 6.6x10 -4 -5 Tartaric HOOC(CHOH)2COOH 9.20x10 4.31x10 Thiocyanic HSCN 0.13 -2 Thiosulfuric H2S2O3 0.3 2.5x10
Trichloroacetic Cl3CCOOH 3 + -10 Trimethyl Ammonium Ion (CH3)3NH 1.58x10
Name Formula Ka1 Ka2 Ka3 -5 Acetic HC2H3O2 1.8*10 -3 -7 -12 Arsenic H3AsO4 5.6*10 1.0*10 3.0*10 -10 Arsenous H3AsO3 6*10 -5 -12 Ascorbic HC6H7O6 8.0*10 1.6*10 -5 Benzoic HC7H5O2 6.5*10 -10 Boric H3BO3 5.8*10 5.6*10- Carbonic H CO 4.3*10-7 2 3 11 -3 Chloroacetic HC2H2O2Cl 1.4*10 -4 -5 -7 Citric H3C6H5O7 7.4*10 1.7*10 4.0*10 Cyanic HCNO 3.5*10-4 -4 Formic HCHO2 1.8*10 -5 Hydroazoic HN3 1.9*10 Hydrocyanic HCN 4.9*10-10 Hydrofluoric HF 6.8*10-4 Hydrogen HCrO - 3.0*10-7 chromate Ion 4 Hydrogen H O 2.4*10-12 peroxide 2 2 Hydrogen HSeO - 2.2*10-2 selenate ion 4 Hydrogen H S 5.7*10-8 1.3*10-13 sulfide 2 Hypobromous HBrO 2*10-9 Hypochlorous HClO 3.0*10-8 Hypoiodous HIO 2*10-11 -1 Iodic HIO3 1.7*10 -4 Lactic HC3H5O3 1.4*10 -3 -6 Malonic H2C3H2O4 1.5*10 2.0*10 -4 Nitrous HNO2 4.5*10 -2 -5 Oxalic H2C2O4 5.9*10 6.4*10 -2 -9 Paraperiodic H5IO6 2.8*10 5.3*10 -10 Phenol HC6H5O 1.3*10 -3 -8 -13 Phosphoric H3PO4 7.5*10 6.2*10 4.2*10 -5 Propionic HC3H5O2 1.3*10 -2 -3 Pyrophosphoric H4P2O7 3.0*10 4.4*10 -3 -9 Selenous H2SeO3 2.3*10 5.3*10 Strong Sulfuric H SO 1.2*10-2 2 4 Acid -2 -8 Sulfurous H2SO3 1.7*10 6.4*10 -3 -5 Tartaric H2C4H4O6 1.0*10 4.6*10 Base Dissociation Constants (25 C)
Name Formula Kb -5 Ammonia NH3 1.8*10 -10 Aniline C6H5NH2 4.3*10 -4 Dimethlyamine (CH3)2NH 5.4*10 -4 Ethylamine C2H5NH2 6.4*10 -6 Hydrazine H2NNH2 1.3*10 -8 Hydroxylamine HONH2 1.1*10 -4 Methylamine CH3NH2 4.4*10 -9 Pyridine C5H5N 1.7*10 -5 Trimethylamine (CH3)CN 6.4*10
question or comments email moneypenny! HOME