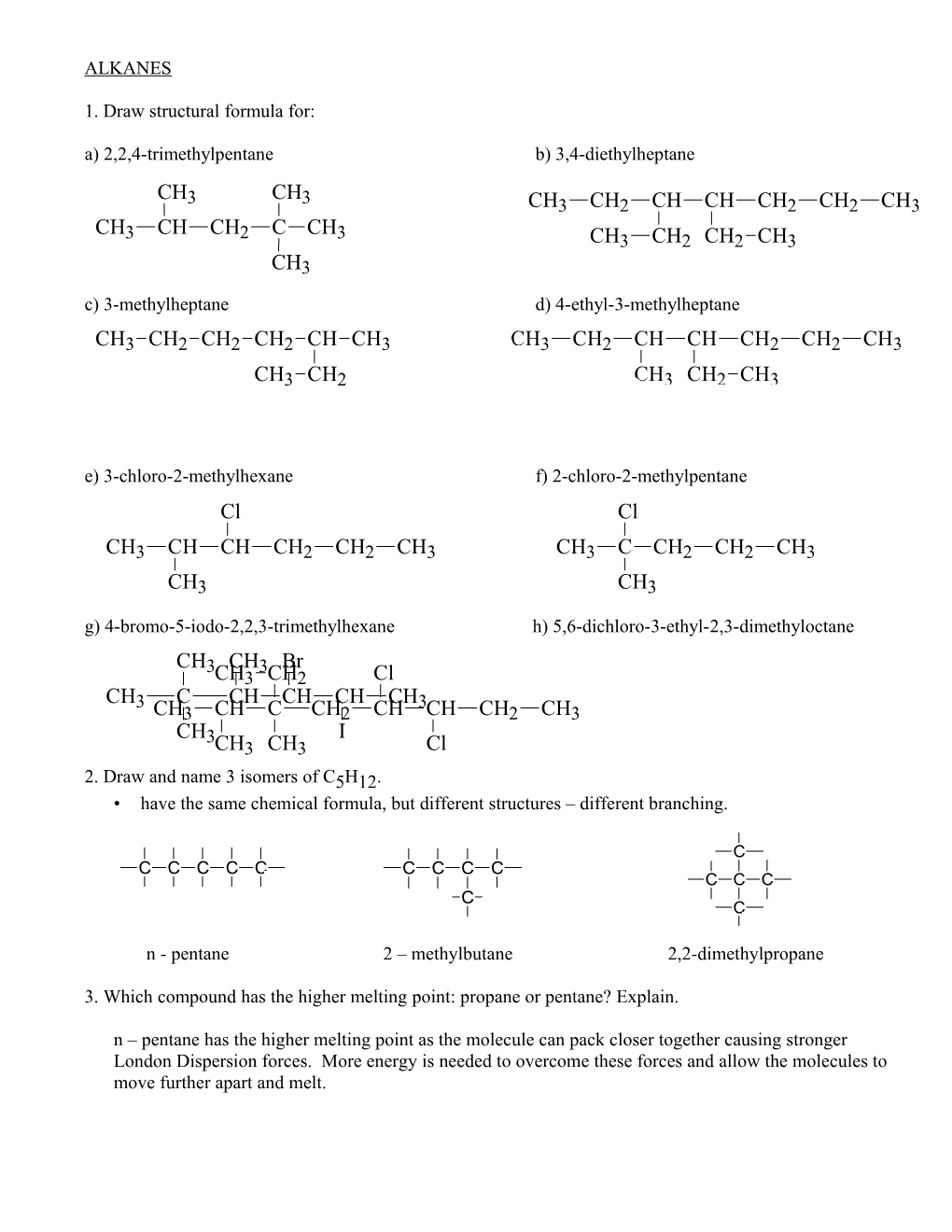
ALKANES
1. Draw structural formula for: a) 2,2,4-trimethylpentane b) 3,4-diethylheptane CH CH 3 3 CH3 CH2 CH CH CH2 CH2 CH3 CH CH CH C CH 3 2 3 CH3 CH2 CH2 CH3 CH3 c) 3-methylheptane d) 4-ethyl-3-methylheptane
CH3 CH2 CH2 CH2 CH CH3 CH3 CH2 CH CH CH2 CH2 CH3 CH3 CH2 CH3 CH2 CH3
e) 3-chloro-2-methylhexane f) 2-chloro-2-methylpentane Cl Cl
CH3 CH CH CH2 CH2 CH3 CH3 C CH2 CH2 CH3 CH3 CH3 g) 4-bromo-5-iodo-2,2,3-trimethylhexane h) 5,6-dichloro-3-ethyl-2,3-dimethyloctane
CH3 CH3 Br CH3 CH2 Cl CH3 C CH CH CH CH3 CH3 CH C CH2 CH CH CH2 CH3 CH3 I CH3 CH3 Cl 2. Draw and name 3 isomers of C5H12. • have the same chemical formula, but different structures – different branching.
C C C C C C C C C C C C C C C
n - pentane 2 – methylbutane 2,2-dimethylpropane
3. Which compound has the higher melting point: propane or pentane? Explain.
n – pentane has the higher melting point as the molecule can pack closer together causing stronger London Dispersion forces. More energy is needed to overcome these forces and allow the molecules to move further apart and melt. ALKENES
1. Draw structural formula for the following: a) 2-methylpropene b) trans-2,3-dibromobut-2-ene
CH3 C CH2 Br CH3 CH3 C C CH3 Br c) cis-hex-3-ene d) 2-ethylpent-1-ene
CH2 CH3 CH2 C C CH2 CH3 CH CH C CH CH CH H H 3 2 2 2 3 e) cis-4-chloro-5-methylhex-2-ene f) 4,4-dimethylhex-1-ene
Cl CH2 CH3 CH CH CH C C CH 3 3 CH3 C CH2 CH CH2 CH H H 3 CH3 g) trans-3-bromo-2-iodo-4,5,5-trimethylhex-2-ene h) trans-3,4-dichloro-6-ethyl-6,7-dimethyloct-3-ene
CH3 CH3 Br CH3 CH2 Cl H3C C CH C C CH3 CH3 CH C CH2 C C CH2 CH3 CH CH Cl CH3 I 3 3
2. a) Draw and name the geometric isomers of C2H2Cl2.
H H H Cl C C C C Cl Cl Cl H cis-1,2-dichloroethane trans-1,2- dichloroethane
b) Which of the two compounds above has the higher melting point? Explain.
cis-1,2-dichloroethane has the higher melting point as it is more polar than the trans isomer. ALKYNES
1. Draw structural formula for the following: a) 4,5-dimethylhept-2-yne b) 3,3-dimethylbut-1-yne CH CH3 C C CH CH CH3 3 HC C C CH CH3 CH2 CH3 3 CH3 c) 4-methylpent-2-yne d) 4-ethylhex-2-yne
CH3 CH2 CH3 CH3 C C CH CH3 CH3 CH2 CH C C CH3 e) 4-chloro-5-methylhex-2-yne f) 5,5-dimethylhept-1-yne
Cl CH2 CH3 CH3 CH CH C C CH3 CH3 C CH2 CH2 C CH CH3 CH3 g) 1-iodo-3,4,4-trimethylpent-1-yne h) 6-ethyl-6,7-dimethyloct-3-yne
CH3 CH3 CH3 CH2 CH3 C CH C C I CH3 CH C CH2 C C CH2 CH3 CH3 CH3 CH3
2. a) Draw and name the isomers of C4H5Cl. There are several, here are a few: H H Cl H H H H H Cl C C C C H H C C C C H H C C C C Cl H C C C C Cl
H H H H H H H H
1-chlorobut-1-yne 3-chlorobut-1-yne 4-chlorobut-1-yne 1-chlorobut-2-yne H H H
H C C C C Cl H C C C C H H C C C C Cl H C C C C H
H H H H H Cl H H H H Cl H H
1-chlorobuta-1,3-diene 3-chlorobuta-1,2-diene 4-chlorobuta-2,3-diene 1-chlorobuta-1,2-diene
b) Which of the isomer compounds above has the higher melting point? Explain.
1-chlorobuta-1,3-diene has the closest packing as it exists in a flat plane.
Nomenclature for Alcohols
Draw the following molecules: a). 2-chlorobutan-2-ol
OH
CH2 C CH3 H3C Cl b) 2-methylheptan-2-ol
C C C C C C C C OH c) octane-1,4-diol
OH
HO CH2 CH CH2 CH3
CH2 CH2 CH2 CH2 d) cyclopentanol
OH
C C C
C C
e) 2,3,4-trichloro-5-methylundecan-1-ol
Cl CH3
CH2 CH CH CH2 CH2 CH3 HO CH CH CH2 CH2 CH2 Cl Cl Nomenclature for Ethers
Draw the following molecules: a) 1-chloro-1-ethoxypropane
C C O C C C
Cl b) 2-methyl-1-propoxyoctane
O CH2 CH2 CH2 H2C CH2 CH2 CH2 CH2
CH CH3 H3C CH3 c) 1-propoxypropane
CH2 O CH2 H3C CH2 CH2 CH3 d) 2-methoxypropane
C C O C
C e) 7-methyoxyoctan-2-ol
CH3
H3C CH2 CH2 CH CH CH2 CH2 O
OH CH3 Nomenclature for Aldehydes
Draw the following molecules: a) 3,3-dimethylbutanal O
CH CH2 CH3 C H3C CH3 b) 4-bromo-3-chloro-5-ethyl-2-methylheptanal
Br CH3
CH2 CH CH O H3C CH CH CH
CH2 Cl H3C c) 2-bromo-3-ethylhexanal Br O C C C C C C
C
C
d) 4-chloropentanal e) 2,2,4-trichlorooctanal Cl O Cl CH C C C C C O C CH CH2 CH3 CH2 CH2 CH2 Cl Cl Nomenclature for Ketones
Draw the following molecules: a) 3-chloro-4-phenylpentan-2-one
CH3 O
HC C CH CH3 Cl b) 3-bromohexan-2-one
O C C C C C C
Br c) 3-ethyl-2,5,7-trimethyloctan-4-one
CH3 CH CH2 HC CH2 3
H3C HC C HC
CH3 O CH CH3
H3C d) 2,2-dimethyldecan-4-one CH3 H3C O C CH3 CH2 CH2 C CH2
H3C CH2 CH2 CH2 e) 1,3,5-trichlorohexane-2,4-dione O Cl C C C C C C Cl Cl O
Nomenclature for Carboxylic Acids
Draw the following molecules: a) 3-fluoropentanoic acid O C C C C C OH
F b) 2,2-dimethylbutanoic acid
C O C C C C OH
C c) 2,3,3,5-tetrachlorooctanoic acid
O Cl HO C C C C C C C C
Cl Cl Cl d) trans-4,4-dimethylhex-2-enoic acid
C O C C C C C C OH
C e) propanedioic acid
O O HO C C C OH
Nomenclature for Esters
Draw the following molecules: a) 1-butyl ethanoate
O C C C C O C C b) 2-butyl propanoate
O C C C O C C C
C c) 3-pentyl 2-methylbutanoate O C C C O C C C C
C C
C
d) 3-methyl-1-butyl propanoate
O C C C O C C C C C
e) dichloromethyl ethanoate
O Cl C C O C Cl
Nomenclature for Amines
Draw the following molecules: a) N-ethyl-1-aminopropane
C C N C C C
b) 2-aminohex-1-ene
N C C C C C C
c) 4-chloro-2-aminoheptane
Cl C C C C C C C
N
d) N-ethyl-N-methyl-2-aminobutane
C C C C
N C C C
e) N-methylaminomethane
C N C
Nomenclature for Amides
Draw the following molecules: a) N-propyl propanamide
O C C C N C C C
b) N-bromo-N-methyl hexanamide
O C C C C C C N C
Br c) N,N-dimethyl pentanamide
O C C C C C N C
C d) 3,N-dimethyl-N-propyl butanamide
O C C C C N C C C
C C e) ethyl-N-methyl butanamide
O C C C C N C
C
C
Name the following compounds:
O 1. 2-methylpropan-2-ol
1-propoxypentane O Br O H3C CH2 C O CH3 10. H3C CH CH2 C OH methyl propanoate 3-bromobutanoic acid 11. O 3. H3C C CH2 CH3 O butanone 3-ethylhexan-2-one
4. H3C CH CH2 OH 12. H3C C C CH3 Cl Cl Br 2-chloropropan-1-ol cis-2-bromo-3-chlorobut-2-ene O 5. H C C C CH CH 3 2 O 13. pent-3-ynal H3C CH2 C N CH2 CH3 Cl 6. HN CH 3 N,N-chloroethyl propanamide H3C C CH2 CH3
CH3 14. H3C CH2 CH2 CH CH3 N-methyl-2-methyl-2-aminobutane O CH2 CH3 7. 2-ethoxypentane O O 2-ethylpentanal 15. H3C C O CH CH2 CH3
H3C 8. H3C CH CH2 CH CH2 CH3 CH3 H2C CH2 CH2 2-butyl ethanoate H3C CH2 16. O 4-ethyl-2-methylnonane OH 9. OH
H3C C CH3
CH3 3-methylhexanoic acid Cl O 17. H3C CH C C CH2 CH
CH3 OH trans-3-chloro-4-hydroxy-5-methylhex-3-enal Comparing Organic Compounds
Draw the following 5 molecules and place them in order of highest boiling point to lowest boiling point. Research their boiling points and confirm your answers. Explain your reasoning fully: a) pentan-1-ol (138 °C) d) pentan-2-one (110 °C) H C CH 3 2 O H3C CH2 CH2 CH2 C CH2 CH2 OH CH3 e) pentanal (103 °C) b) 2-methylbutan-2-ol (102 °C) CH3 H3C CH2 CH C CH2 CH2 O 2 CH3 H3C CH2 CH HO c) 1-ethoxypropane (64 °C)
H3C O CH2 CH2 CH2 CH3
Draw the following 7 molecules and place them in order of highest boiling point to lowest boiling point. Explain your reasoning: c) hexanoic acid (204 °C) a) hexan-3-ol (135 °C)
O OH
CH2 CH2 C H3C CH CH2 H3C CH2 CH2 OH CH2 CH2 CH3 d) hexan-3-one (123 °C) e) 1-propyl propanoate (123 °C)
O O CH H3C C CH2 H3C CH2 C 3 CH2 CH2 CH3 CH2 O CH2 g) 3-aminohexane (115 °C) b) 1-methoxy-2-methylbutane (81 °C) NH2 CH3
H3C CH CH2 H3C CH O CH2 CH2 CH3 CH2 CH2 CH3 f) 2-methylpentane (69 °C) H3C CH2 CH3 CH2 CH
CH3 Organic Reactions Worksheet
Note – Use HCl, H2O, OA such as FeO, CuO, KMnO4,etc. and NH3,, Cl2 when necessary
1. Using an alkene, produce butan-2-one
Need: 2° alcohol; butan-2-ol
C C C C + H2O C C C C OH
but-1-ene butan-2-ol addition
O
C C C C + FeO C C C C + H2O + Fe OH
butan-2-ol butanone oxidation
2. Using two alcohols, produce methyl butanoate
Need: methanol and butanoic acid
Create the carboxylic acid: O
C C C C OH + CuO C C C C + H2O + Cu
butan-1-ol butanal oxidation
O O
C C C C + CuO C C C C OH + H2O + Cu
butanal butanoic acid oxidation Create the ester: O O C C C C OH + HO C C C C C O C + Cu
butanoic acid methyl butanoate condensation
3. Using an alkane and an carboxylic acid, produce N-ethyl butanamide
Need: aminoethane and butanoic acid
Create the amine: C C + Cl Cl C C Cl + HCl
ethane 1-chloroethane substitution
C C Cl + NH3 N C C + HCl
1-chloroethane aminoethane substitution
Create the amide: O O C C C C OH + N C C C C C C N C C + H2O
butanoic acid aminoethane N-ethyl butanamide condensation
4. Using an alkane and an alkene, produce methoxyethane
Need: aminoethane and butanoic acid
Create an alcohol from the alkane:
C + Cl Cl C Cl + HCl
methane chloromethane substitution
C Cl + H2O C OH + HCl
chloromethane methanol substitution
Create an alcohol from the alkene:
C C + H2O C C OH
ethane ethanol addition
Create the ether:
C OH + HO C C C O C C + H2O
methanol ethanol methoxyethane condensation
5. Using an alcohol and an haloalkane, produce ethyl propanoate
Need: ethanol and propanoic acid
Create the carboxylic acid from the alcohol: O
C C C OH + MnO2 C C C + H2O + MnO
propan-1-ol propanal oxidation O O C C C + MnO C C C OH + Mn
propanal propanoic acid oxidation
Create the alcohol from the haloalkane:
C C Cl + H2O C C OH + HCl
chloroethane ethanol substitution
Create the ester: O O
C C C OH + HO C C C C C O C C + H2O
propanoic acid ethanol ethyl propanoate condensation
6. Using an alcohol, produce an aminoethane
Need: ethanol
Create an alkene from the alcohol:
H2SO4 C C OH C C + H2O
ethanol ethene elimination
Create the haloalkane:
C C + HCl C C Cl
ethene chloroethane substitution
C C Cl + NH3 C C N + HCl
chloroethane aminoethane substitution
7. Using an alkene, produce pentan-2-one
Need: pentan-2-ol
C C C C C + H2O C C C C C OH pent-1-ene pentan-2-ol addition
O
C C C C C + ZnO C C C C C + H2O + Zn OH pentan-2-ol pentan-2-one oxidation ALIPHATIC HYDROCARBON ASSIGNMENT
1. (a) Look up the boiling points for the alkanes, methane through decane.
C1 -164°C C2 -89°C C3 -42°C C4 -0°C C5 31°C C6 69°C C7 98°C C8 126°C C9 151°C C10 174°C
(b) Explain the trend in their boiling points.
As the C chain length increases, the number of electrons increase and the London Dispersion Force increases due to the greater temporary dipoles.
2. (a) Lookup the boiling points for 2-methyl pentane and 2,2-dimethyl butane.
60.3°C 49.7°C
(b) Draw their structural formulae.
C C C C C C C C C C C C
(c) Name their straight chain alkane isomer.
Hexane
(d) Explain the trend in boiling points within this series of isomers.
Branching increases distance of closest approach between molecules and therefore weakens interparticle forces. Branching decreases magnitude of temporary dipoles and therefore weakens London Dispersion forces.
3. Explain why reactions involving alkanes are often quite slow in spite of being significantly exothermic.
Collision geometry is complex and the mechanism will involve the breakage of numerous strong covalent bonds. The C’s have a negative oxidation number which stabilizes the atoms and makes it more difficult to break the strong bonds. 4. Construct models, give structural formulae and condensed formulae and IUPAC names for as many isomers of C6H12 as possible. Use straight chain branches only.
Answers variable and would include
C C C C C C CH2CHCH2CH2CH2CH3 hexene
C C C C C C CH3CHCHCH2CH2CH3 2-hexene
C
C C C C C CH3CHC(CH3)CH2CH3 3-methyl-2-pentene
6. Give structural and projection formulae for the geometric isomers of 3-hexene.
H H H H H H C H C H H C H C H H C C H H H C C H C C H H C H H H H C H H CIS TRANS
7. Using structural formulae, illustrate the following reactions (a) propane + chlorine
(b) but-1-yne + oxygen (c) but-1-ene + hydrochloric acid
C C C C + HCl C C C C Cl SOME FUNCTIONAL GROUPS AND THEIR REACTIVITY
1. List the functional groups for the following classes of compounds; alcohols, ethers aldahydes, ketones, carboxylic acids, esters, amines and amides.
Common groups of atoms which result in common properties and allow classification of compounds into groups with similar properties.
2. For each of the following sets of compounds and their boiling points (ii) Name the compounds and (ii) Explain the trend in boiling point.
(a) CH4 (-164 °C) HCHO (-21 °C) CH3OH (65 °C) HCO2H (101 °C)
methane methanal methanol methanoic acid
London dispersion, dipole-dipole, H-bonding, stronger H-bonding due to degree of polarity
(b) CH3OH (65 °C) C2H5OH (79 °C) C3H7OH (98 °C)
methanol ethanol propan-1-ol
Larger molecules with more electrons, result in larger London dispersion and dipole- dipole forces
(c) C2H6 (-89 °C) CH3OCH3 (-25 °C) C2H5OC2H5 (35 °C)
ethane methoxymethane ethoxyethane
London dispersion, slight dipole-dipole and London dispersion forces, slightly larger dipole-dipole and dispersion forces due to greater size and number of electrons.
(d) C3H8 (-42 °C) CH3COCH3 (56 °C) C2H5COC2H5 (102 °C)
propane propanone pentan-3-one
London dispersion, dipole-dipole, dipole-dipole with larger London dispersion forces due to greater size and more electrons. (e) CH3CO2H (118 °C) C2H5CO2H (141 °C) C3H7CO2H (164 °C)
ethanoic acid propanoic acid butanoic acid
Stronger H-bonding & increasing London dispersion forces due to greater size and number of electrons (f) CH3CO2CH3 (57 °C) C2H5CO2C2H5 (99 °C)
methyl ethanoate ethyl propanoate
Increasing dipole-dipole and London dispersion forces due to greater size and number of electrons.
3. Give the structural diagrams, condensed formulae and the names for the primary, secondary and tertiary isomers of C6H13OH.
H H H H H H
H C C C C C C O H
H H H H H H CH3-CH2-CH2-CH2-CH2-CH2-OH hexan-1-ol H H H H H H
H C C C C C C H
H H H H OH H CH3-CH2-CH2-CH2-CH(OH)-CH3 hexan-2-ol H
H C H
H H H H
H C C C C C H
H H H OH H CH3-CH2-CH2-C(CH3)(OH)-CH3 2 methylpentan-2-ol
4. Using an alcohol with a two carbon main chain, illustrate end explain the differences in oxidation of primary, secondary and tertiary alcohols. In each case, name the product of the reaction and use oxidation numbers to confirm that oxidation has occurred.
Primary: - M nO O 4 + - C C OH C C + 2 H + 2 e H -1 +1 ethanol ethanol - O M nO O 4 + - C C C C + 2 H + 2 e H OH +1 +3 ethanal ethanoic acid Secondary:
C - C O M nO 4 + - C C C C C + 2 H + 2 e O C H
0 +2 propan-2-ol propanone
Tertiary: No further oxidation due to the lack of any remaining hydrogen atoms on the functional group carbon.
5. Illustrate using structural formulae and balanced equations, each of the following. Name all products and types of reaction.
(a) methanoic acid + water
H H - H C H C + OH + H2O O + H3O H H
(b) 2-methylbutanoic acid + sodium carbonate
H H H O H H H O
H C C C C OH + Na2CO3 H C C C C O Na + CO2 + H2O H H H H CH3 CH3 (c) ethanoic acid + propan-1-ol
O O H+ C C + H O C C C C C O C C C + H2O OH
(d) propyl ethanoate + sulfuric acid
O O H2SO4 + H O C C C C C O C C C + H2O C C OH