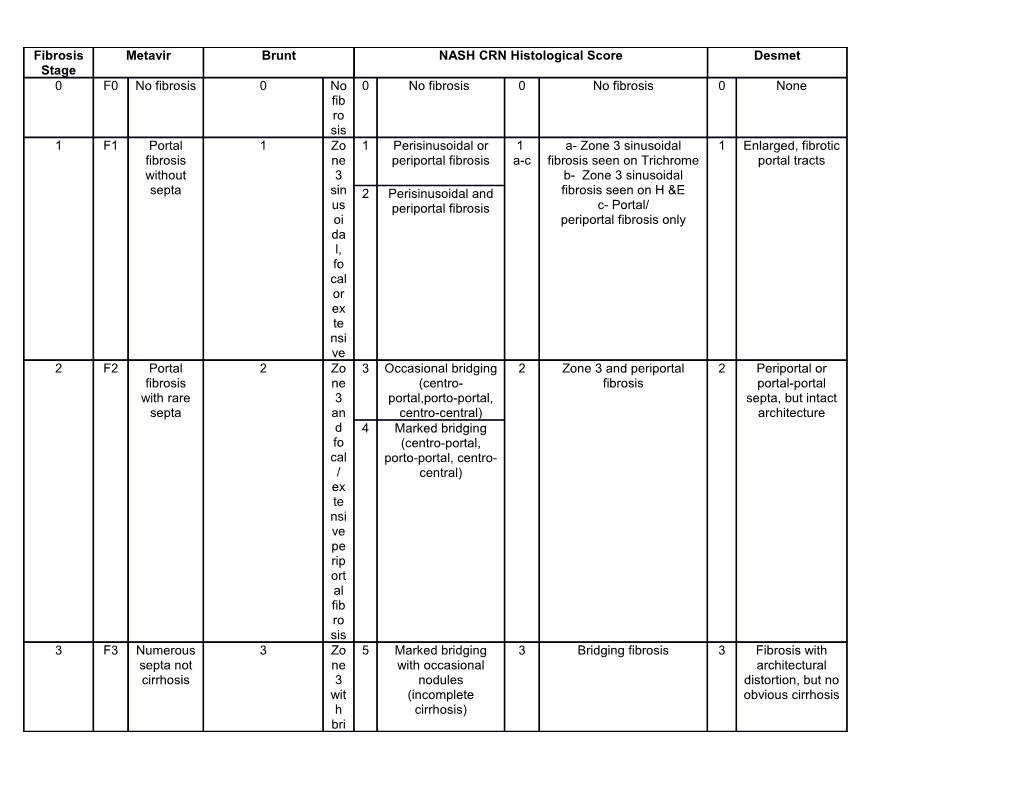
Fibrosis Metavir Brunt NASH CRN Histological Score Desmet Stage 0 F0 No fibrosis 0 No 0 No fibrosis 0 No fibrosis 0 None fib ro sis 1 F1 Portal 1 Zo 1 Perisinusoidal or 1 a- Zone 3 sinusoidal 1 Enlarged, fibrotic fibrosis ne periportal fibrosis a-c fibrosis seen on Trichrome portal tracts without 3 b- Zone 3 sinusoidal septa sin 2 Perisinusoidal and fibrosis seen on H &E us periportal fibrosis c- Portal/ oi periportal fibrosis only da l, fo cal or ex te nsi ve 2 F2 Portal 2 Zo 3 Occasional bridging 2 Zone 3 and periportal 2 Periportal or fibrosis ne (centro- fibrosis portal-portal with rare 3 portal,porto-portal, septa, but intact septa an centro-central) architecture d 4 Marked bridging fo (centro-portal, cal porto-portal, centro- / central) ex te nsi ve pe rip ort al fib ro sis 3 F3 Numerous 3 Zo 5 Marked bridging 3 Bridging fibrosis 3 Fibrosis with septa not ne with occasional architectural cirrhosis 3 nodules distortion, but no wit (incomplete obvious cirrhosis h cirrhosis) bri dg in g fib ro sis fro m zo ne 3 to 1 wit h no du lar ch an ge 4 F4 Cirrhosis 4 Cir 6 Cirrhosis, probable 4 Cirrhosis 4 Probable or rh or definitive definite cirrhosis osi s
Supplementary Table 1. Scheme for reconciling all fibrosis stages (for different etiologies of chronic liver disease) into a comparable 5-stage system used in our pooled analysis Y C W R L Asbac A L i h a u o h 2008 s o n e n s w b o n g t a m o c b QUADAS Assessment Items g h a i 2 0 1 0 1. Was the spectrum of patients representative of the patients N Y Y Y N N N Y who will receive the test in practice? [Spectrum Bias] 2. Were selection criteria clearly described? Y Y Y Y N Y Y Y 3. Is the reference standard likely to correctly classify the Y Y Y Y Y Y Y Y target condition? 4. Is the time period between reference standard and index test short enough to be reasonably sure that the target Y Y Y Y Y Y Y Y condition did not change between the two tests? [Disease Progression Bias] 5. Did the whole sample or a random selection of the sample, receive verification using a reference standard of diagnosis? Y Y Y Y Y Y Y Y [Partial Verification Bias] 6. Did patients receive the same reference standard regardless Y U Y Y Y Y Y Y of the index test result? [Differential Verification Bias] 7. Was the reference standard independent of the index test (i.e. the index test did not form part of the reference Y Y Y Y Y Y Y Y standard)? [Incorporation Bias] 8. Was the execution of the index test described in sufficient Y Y Y Y N Y Y Y detail to permit replication of the test? 9. Was the execution of the reference standard described in Y Y Y Y N N Y Y sufficient detail to permit its replication? 10. Were the index test results interpreted without knowledge of Y Y Y Y U N Y Y the results of the reference standard? [Review Bias] 11. Was the reference standard results interpreted without Y Y Y Y U Y Y Y knowledge of the results of the index test? [Review Bias] 12. Were the same clinical data available when test results were interpreted as would be available when the test is used in Y Y Y Y N N N Y practice? 13. Were uninterpretable/indeterminate test results reported? Y Y Y Y Y Y Y Y 14. Were withdrawals from the study explained? Y Y Y Y Y Y Y Y Total Score (Max=14) 13 13 14 14 7 10 12 14 Supplementary Table 2. Methodological and reporting quality of the included studies using quality assessment of diagnostic accuracy studies (QUADAS) questionnaire. Search Strategy
Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations and Ovid MEDLINE(R) 1946 to Present Search # Searches Results Type elasticity/ or elasticity imaging techniques/ or elastography.mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword 1 34012 Advanced heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] 2 magnetic resonance imaging/ or diffusion magnetic resonance imaging/ 291772 Advanced 1 and (2 or mre.mp.) [mp=title, abstract, original title, name of substance word, 3 subject heading word, keyword heading word, protocol supplementary concept, 858 Advanced rare disease supplementary concept, unique identifier] 4 Liver/pa, ra, ri [Pathology, Radiography, Radionuclide Imaging] 74251 Advanced 4 and (fibrosis or fibrotic or stiff*).mp. [mp=title, abstract, original title, name of 5 substance word, subject heading word, keyword heading word, protocol 7514 Advanced supplementary concept, rare disease supplementary concept, unique identifier] 6 3 and 5 37 Advanced exp Liver Diseases/di, pa, pp, ra, ri [Diagnosis, Pathology, Physiopathology, 7 160780 Advanced Radiography, Radionuclide Imaging] 8 exp liver cirrhosis/ or 7 or 4 242766 Advanced 9 3 and 8 108 Advanced 10 6 or 9 108 Advanced 10 and systematic*.mp. [mp=title, abstract, original title, name of substance word, 11 subject heading word, keyword heading word, protocol supplementary concept, 11 Advanced rare disease supplementary concept, unique identifier] limit 10 to (clinical trial, all or clinical trial, phase i or clinical trial, phase ii or clinical trial, phase iii or clinical trial, phase iv or clinical trial or comparative study 12 or controlled clinical trial or evaluation studies or meta analysis or multicenter 57 Advanced study or randomized controlled trial or "review" or systematic reviews or validation studies) 3 and 8 and (exp biopsy/ or histopatholog*.mp.) [mp=title, abstract, original title, 13 name of substance word, subject heading word, keyword heading word, protocol 34 Advanced supplementary concept, rare disease supplementary concept, unique identifier] 10 and ("sensitivity and specificity"/ or predictive value of tests/ or reproducibility 14 35 Advanced of results/) 15 exp Diagnostic Errors/ 94620 Advanced 16 exp markov chains/ or exp uncertainty/ or exp "sensitivity and specificity"/ 435911 Advanced 17 exp area under curve/ 27563 Advanced 18 10 and (15 or 16 or 17) 33 Advanced 19 6 or 11 or 12 or 13 or 14 or 18 94 Advanced 20 remove duplicates from 19 73
Embase 1988 to 2013 Week 38 Search # Searches Results Type elasticity/ or elasticity imaging techniques/ or elastography.mp. [mp=title, 1 abstract, subject headings, heading word, drug trade name, original title, device 25056 Advanced manufacturer, drug manufacturer, device trade name, keyword] 2 magnetic resonance imaging/ or diffusion magnetic resonance imaging/ 444067 Advanced 1 and (2 or mre.mp.) [mp=title, abstract, subject headings, heading word, drug 3 trade name, original title, device manufacturer, drug manufacturer, device trade 1288 Advanced name, keyword] 4 [Liver/pa, ra, ri [Pathology, Radiography, Radionuclide Imaging]] 0 Advanced 4 and (fibrosis or fibrotic or stiff*).mp. [mp=title, abstract, subject headings, 5 heading word, drug trade name, original title, device manufacturer, drug 0 Advanced manufacturer, device trade name, keyword] 6 3 and 5 0 Advanced 7 [exp Liver Diseases/di, pa, pp, ra, ri [Diagnosis, Pathology, Physiopathology, 0 Advanced Radiography, Radionuclide Imaging]] 8 exp liver cirrhosis/ or 7 or 4 76199 Advanced 9 3 and 8 131 Advanced 10 6 or 9 131 Advanced 10 and systematic*.mp. [mp=title, abstract, subject headings, heading word, drug 11 trade name, original title, device manufacturer, drug manufacturer, device trade 9 Advanced name, keyword] limit 10 to (clinical trial, all or clinical trial, phase i or clinical trial, phase ii or clinical trial, phase iii or clinical trial, phase iv or clinical trial or comparative 12 study or controlled clinical trial or evaluation studies or meta analysis or 43 Advanced multicenter study or randomized controlled trial or "review" or systematic reviews or validation studies) [Limit not valid in Embase; records were retained] 3 and 8 and (exp biopsy/ or histopatholog*.mp.) [mp=title, abstract, subject 13 headings, heading word, drug trade name, original title, device manufacturer, drug85 Advanced manufacturer, device trade name, keyword] 10 and ("sensitivity and specificity"/ or predictive value of tests/ or reproducibility 14 39 Advanced of results/) 15 exp Diagnostic Errors/ 50160 Advanced 16 exp markov chains/ or exp uncertainty/ or exp "sensitivity and specificity"/ 254035 Advanced 17 exp area under curve/ 67293 Advanced 18 10 and (15 or 16 or 17) 33 Advanced exp case control study/ or exp case study/ or exp clinical trial/ or exp intervention 19 study/ or exp major clinical study/ or exp prospective study/ or exp retrospective 2684494 Advanced study/ 20 exp predictive value/ 40920 Advanced 21 area under the curve/ 67293 Advanced 22 receiver operating characteristic/ 33260 Advanced 23 diagnostic accuracy/ 173460 Advanced 10 and (19 or 20 or 21 or 22 or 23 or staging*.mp.) [mp=title, abstract, subject 24 headings, heading word, drug trade name, original title, device manufacturer, drug66 Advanced manufacturer, device trade name, keyword] 25 liver fibrosis/ 20869 Advanced 26 3 and 25 219 Advanced 26 and (19 or 20 or 21 or 22 or 23 or exp biopsy/ or histopathol*.mp.) [mp=title, 27 abstract, subject headings, heading word, drug trade name, original title, device 179 Advanced manufacturer, drug manufacturer, device trade name, keyword] 28 18 or 24 or 27 197
Web of Science Topic=((elasticity OR elastography OR viscoelasticity OR stiffness) AND (mre OR mr OR "magnetic resonance")) AND Topic=((cirrhosis OR cirrhotic OR fibrosis OR fibrotic) OR liver OR hepat*) AND Topic=("area under" OR roc OR reproducib* OR accura* OR predictive OR value OR sensitiv* OR compar* OR biops* OR histopathol* OR "systematic review" OR meta-analysis) 293
Scopus (TITLE-ABS-KEY((elasticity OR elastography OR viscoelasticity OR stiffness) AND (mre OR mr OR "magnetic resonance")) AND TITLE-ABS-KEY((cirrhosis OR cirrhotic OR fibrosis OR fibrotic OR liver OR hepat*)) AND TITLE-ABS-KEY((staging OR "area under" OR roc OR reproducib* OR accura* OR predictive OR value OR sensitiv* OR compar* OR biops* OR histopathol* OR "systematic review" OR meta-analysis))) 426