Unit 3 Chemistry – Functional Groups in hydrocarbons [to C10]
Apart from bonding to itself or hydrogen, carbon can also form covalent bonds with other atoms or groups of atoms. These atoms or groups of atoms, that give the hydrocarbon a characteristic set of properties, are called functional groups.
functional formula found in semi-structural formula of hydrocarbon group
chloro R–Cl chloroalkanes CH 3 CH 2CH2Cl 1-chloropropane
hydroxy R–OH alkanols CH3CH2OH ethanol
carboxy R–COOH carboxylic acids CH3CH2CH2COOH butanoic acid
amino R–NH2 amines CH3CH2NH2 aminoethane
ester R–OCO–R’ esters CH3CH2COOCH3 methypropanoate
amido R–CONH–R’ amides CH3CH2CONH2 ethanamide
ether R–O–R’ ethers CH3CH2OCH2CH3 diethylether
Note: in the general formula R-OH, the R represents an alkyl group, as in
R = ethyl [CH3CH2–] in ethanol [CH3CH2OH]
Chloroalkanes it is possible to replace a –H with a –Cl to form a chloroalkane the name of a chloroalkane starts with “chloro” followed by the name of the alkane, with the position of the chloro is given by numbering …
eg. CH3CHClCH3 = 2-chloropropane more than one –H can be replaced with a –Cl …
eg. CH3CH2CHClCH2CHClCH3 = 2,4-dichlorohexane other halogens [ F, Br, I ] form similar molecules with alkanes
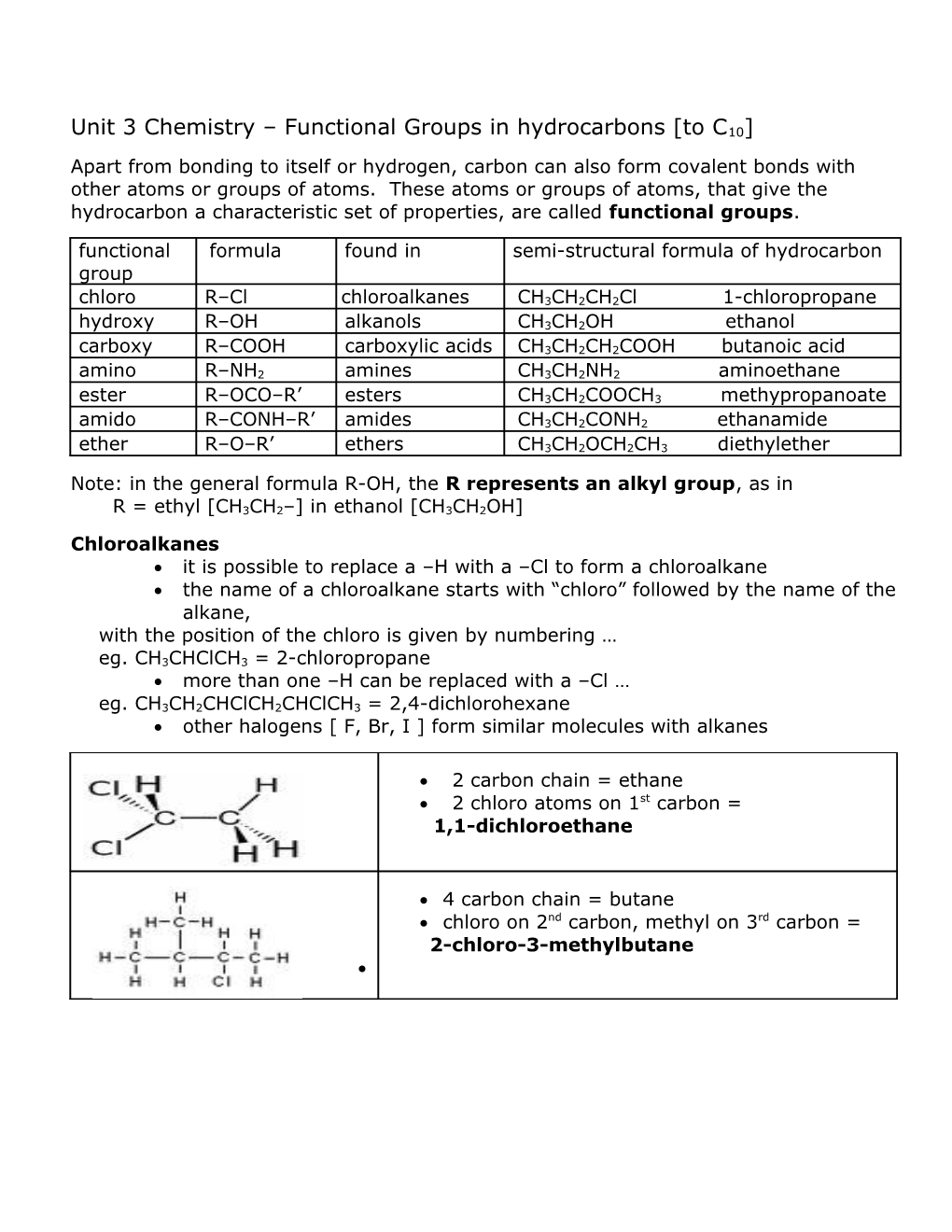
2 carbon chain = ethane 2 chloro atoms on 1st carbon = 1,1-dichloroethane
4 carbon chain = butane chloro on 2nd carbon, methyl on 3rd carbon = 2-chloro-3-methylbutane
4 carbon chain = butane bromo and chloro on 2nd carbon, chloro on 3rd carbon = 2-bromo-2,3-dichlorobutane
Alkanols in alkanols (or alcohols) with a general formula R–OH, a hydrogen on the alkane has been replaced by a hydroxy group [–OH] hydrogen bonding due to the polar –OH group, give alkanols a higher boiling point and greater water solubility than their corresponding alkane alkanols are named by replacing the “e” at the end of the alkane and replacing it with “ol” eg. ethane becomes ethanol the position of the –OH in the alkane is indicated by numbering
eg. CH3CH2CHOHCH3 = butan-2-ol more than one –H can be replaced with a –OH …
eg. HOCH2CH2OH = ethan-1,2-diol
3 carbon chain = propane hydroxy [–OH] group on 2nd carbon = propan-2-ol
4 carbon chain = butane hydroxy on 2nd carbon = butan-2-ol 2 methyl groups on 3rd carbon = 3,3-dimethylbutan-2-ol
3 carbon chain = propane 3 hydroxy groups, on 1st, 2nd and 3rd carbon = propan-1,2,3-triol [ = glycerol ]
Carboxylic acids carboxylic acids, with the general formula R-COOH contain the carboxy functional group [ –COOH ] the carboxy group is always at one end of the carboxylic acid the tendency of the carboxy group [–COOH] to donate its H+ gives these hydrocarbons their weak acidity; the polarity of the bonds in the carboxy group make smaller carboxylic acids soluble in water to name a carboxylic acid, the number of carbons [ including the carboxy carbon ] in the longest chain has the “e” replaced by “oic acid”
eg. CH3CH2COOH = propanoic acid
4 carbon chain = butane carboxy group at end = butanoic acid
6 carbon chain = hexane carboxy group at end = hexanoic acid rd methyl group on 3 carbon = 3-methylhexanoic acid
5 carbon chain = pentane carboxy group at end = pentanoic acid ethyl group on 2nd carbon = 2-ethylpentanoic acid
Amines
amines, with the general formula R–NH2, have the amino [–NH2] functional group the polarity of the N–H bonds in the amino group make smaller amines water soluble the amines are named by adding “amino” to the front of the alkane name …
eg. CH3CH2CH2NH2 = aminopropane
the the position of the – NH2 in the amine is indicated by numbering …
eg. CH3CH2CHNH2CH3 = 2-aminobutane
2 carbon chain = ethane amino group attached = aminoethane
5 carbon chain = pentane amino group on 2nd carbon = 2-aminopentane
Amino acids amino acids are the basic building blocks of proteins amino acids contain the amine and the carboxylic acid functional groups
alanine – an -amino acid, has an –NH2 amino functional group and a –COOH carboxylic acid functional group attached to the same carbon
Functional Groups – Structure and Naming Copy each of these structural formula and then for each one:- (a) identify the group of compounds it belongs to [eg. carboxylic acid] (b) highlight and label its functional group (c) systematically name it
Remember: In these structures, if omitted, there is a carbon atom at each intersection of lines and at each free end of the line