Electrolysis of Solutions
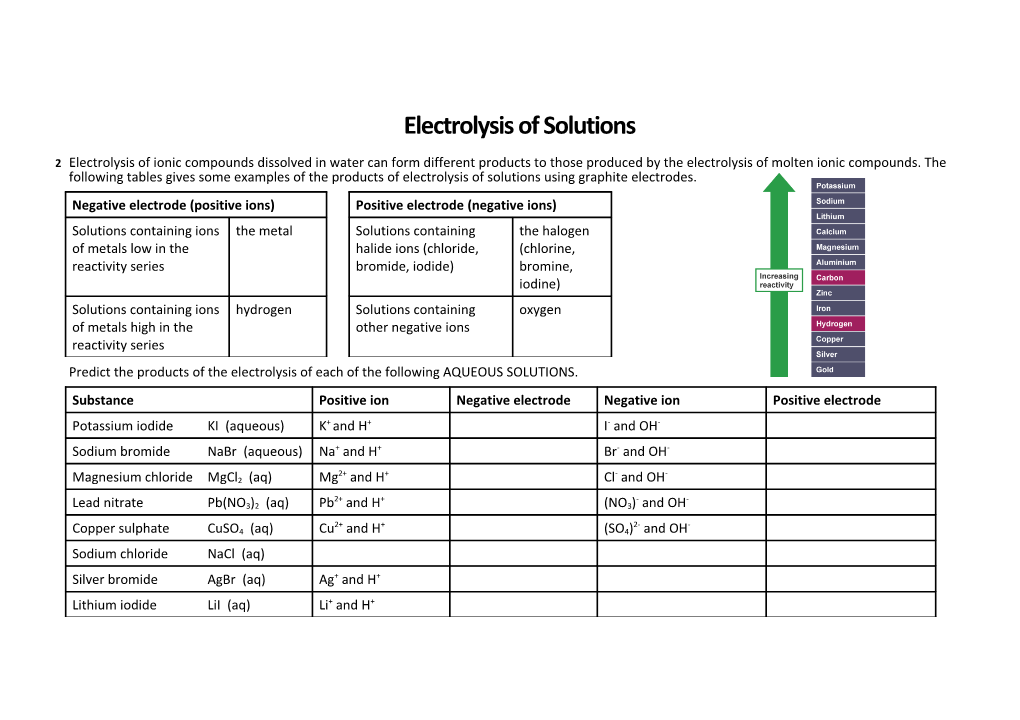
2 Electrolysis of ionic compounds dissolved in water can form different products to those produced by the electrolysis of molten ionic compounds. The following tables gives some examples of the products of electrolysis of solutions using graphite electrodes. Negative electrode (positive ions) Positive electrode (negative ions) Solutions containing ions the metal Solutions containing the halogen of metals low in the halide ions (chloride, (chlorine, reactivity series bromide, iodide) bromine, iodine) Solutions containing ions hydrogen Solutions containing oxygen of metals high in the other negative ions reactivity series Predict the products of the electrolysis of each of the following AQUEOUS SOLUTIONS. Substance Positive ion Negative electrode Negative ion Positive electrode
+ + - - Potassium iodide KI (aqueous) K and H I and OH Sodium bromide NaBr (aqueous) Na+ and H+ Br- and OH-
2+ + - - Magnesium chloride MgCl2 (aq) Mg and H Cl and OH
2+ + - - Lead nitrate Pb(NO3)2 (aq) Pb and H (NO3) and OH
2+ + 2- - Copper sulphate CuSO4 (aq) Cu and H (SO4) and OH Sodium chloride NaCl (aq) Silver bromide AgBr (aq) Ag+ and H+ Lithium iodide LiI (aq) Li+ and H+ Electrolysis of Molten Salts
1 Electrolysis of pure ionic compounds form the elements of the ions that they are made up of. The following tables gives some examples of the products of electrolysis of molten salts using graphite electrodes. Negative electrode (positive ions) Positive electrode (negative ions) Molten salts containing the metal Molten salts containing the non-metal ions of a metal non-metal group 7 group 7 halogen ions halogen ion (chlorine, bromine, iodine) Solutions containing ions hydrogen Molten salt with other the non-metal of hydrogen (H+) non-metal elements Predict the products of the electrolysis of each of the following MOLTEN ELECTROLYTES. Substance Positive ion Negative electrode Negative ion Positive electrode Potassium iodide KI (liquid) K+ I- Sodium bromide NaBr (liquid) Na+ Br-
2+ - Magnesium chloride MgCl2 (l) Mg Cl
2+ 3- Lead (II) nitride Pb3N2 (l) Pb N Copper sulphide CuS (l) Cu2+ S2- Sodium chloride NaCl (l) Silver bromide AgBr (l) Ag+ Lithium iodide LiI (l) Li+