26 August 2021 Aperto
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2020 Dicembre Ok.Cdr
GIORNALE DI INFORMAZIONE DEL COMUNE DI CASTIGLIONE TINELLA - DICEMBRE 2020 NELL’ATTESA LA VITA CONTINUA Sta per concludersi un anno che vorremmo tutti dimenticare, invece rimarrà siamo imposti. Così come le nostre cantine sono raggruppate e rappresentate nella memoria e nella storia di questo nostro paese come un anno infausto, dalla Bottega del vino Moscato, le altre piccole attività avranno una attenzione e colpito come tutta la Nazione da un evento straordinario che distorce la vita una rappresentanza attraverso una delega comunale dedicata. Ma questo è solo quotidiana e arriva a fare vittime. Il Covid-19 ha avuto la sua vittima anche nel uno dei tanti argomenti contenuti nel programma di lavoro presentato, nostro Comune, diverse altre persone sono state contagiate fortunatamente elemento di continuità dell’impegno svolto finora. Vogliamo da oggi senza grossi problemi di salute mentre alcuni hanno dovuto sottoporsi a un approfondire anche la conoscenza di quelle persone che sono giunte a semplice isolamento forzato (lungo questo anno, finora i castiglionesi risultati comprare casa da noi da ogni angolo del mondo: un’attività particolarmente positivi al tampone sono stati 40). Come è stato in primavera, anche la nostra importante per Castiglione, che contribuisce allo sviluppo sociale e che offre Residenza S. Andrea ha avuto nuovamente notevoli difficoltà ad affrontare il affascinanti aperture verso il mondo da famiglie innamorate del nostro contagio, nonostante la grande territorio. Scoprirete su queste pagine attenzione e professionalità che da e anche su quelle web del sito sempre caratterizzano il lavoro degli comunale lo sviluppo dei tanti operatori. Ora la situazione pare offrire progetti; l’ultimo Consiglio comunale, positive aperture e la notizia del vaccino svolto per la prima volta nella nostra che dovrebbe essere distribuito nei storia in videocollegamento, ha prossimi mesi lascia sperare in orizzonti decretato un equilibrio di bilancio: i sereni. -

Brus Robiola D'alba Nostrale D'alpe Sola Toumin Dal Mel Paglierina
Nostrale Toumin d’Alpe dal Mel Brus ow’s cheese, wholly or slightlyskimmed from the top. Pres- fresh soft-textured cheese that matures in around a term that cannot be translated into Italian. It derives Csed when fresh, it is produced exclusively with Alpine milk. Aweek. Produced all year round from cow’s milk and less A from “bruciare” (burn) the vigorous sensation that as- Cylindrical, diameter 25-40 cm, rather squat and slightly convex than 10% goat’s milk. Cylindrical in shape. Diameter 10-12 cm, saults the mouth at first taste. It may be written as brôz sides, it weighs 3-8 kg. Fine and elastic crust, grey-straw colour notably convex sides. Weighs 150-200 grams. or bruz, but the fire remains! Pungent aroma and enticing when less mature and hard, thick and dark brown in the more The fresh product is without a crust. When matured it deve- perfume. In reality it is an amalgam of many cheeses redu- mature varieties. The texture and taste vary according the age: lops a fine layer of white mould than is completely edible. ced to cream with a small quantity of fresh milk that is left the fresh product is soft and clean to cut with small holes and The taste-aroma varies from sweet and delicate in the fresh to ferment for a number of days. An ancient story, based a sweet and aromatic taste; the more mature variety is compact varieties to a pronounced taste in those matured for a number on sobriety and frugality. It proudly reigns over the autumn and coarse-grained with a more or less strong straw colour and of days. -
Zone Del Sistema Confartigianato Cuneo -> Comuni
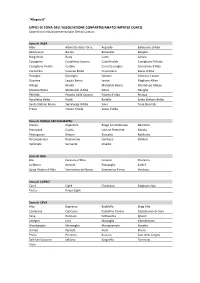
“Allegato B” UFFICI DI ZONA DELL’ASSOCIAZIONE CONFARTIGIANATO IMPRESE CUNEO Zone e loro limitazione territoriale. Elenco Comuni. Zona di ALBA Alba Albaretto della Torre Arguello Baldissero d’Alba Barbaresco Barolo Benevello Bergolo Borgomale Bosia Camo Canale Castagnito Castelletto Uzzone Castellinaldo Castiglione Falletto Castiglione Tinella Castino Cerretto Langhe Corneliano d’Alba Cortemilia Cossano Belbo Cravanzana Diano d’Alba Feisoglio Gorzegno Govone Grinzane Cavour Guarene Lequio Berria Levice Magliano Alfieri Mango Montà Montaldo Roero Montelupo Albese Monteu Roero Monticello d’Alba Neive Neviglie Perletto Pezzolo Valle Uzzone Piobesi d’Alba Priocca Rocchetta Belbo Roddi Rodello Santo Stefano Belbo Santo Stefano Roero Serralunga d’Alba Sinio Tone Bormida Treiso Trezzo Tinella Vezza d’Alba Zona di BORGO SAN DALMAZZO Aisone Argentera Borgo San Dalmazzo Demonte Entracque Gaiola Limone Piemonte Moiola Pietraporzio Rittana Roaschia Robilante Roccasparvera Roccavione Sambuco Valdieri Valloriate Vernante Vinadio Zona di BRA Bra Ceresole d’Alba Cervere Cherasco La Morra Narzole Pocapaglia Sanfrè Santa Vittoria d’Alba Sommariva del Bosco Sommariva Perno Verduno Zona di CARRÙ Carrù Cigliè Clavesana Magliano Alpi Piozzo Rocca Cigliè Zona di CEVA Alto Bagnasco Battifollo Briga Alta Camerana Caprauna Castellino Tanaro Castelnuovo di Ceva Ceva Garessio Gottasecca Igliano Lesegno Lisio Marsaglia Mombarcaro Mombasiglio Monesiglio Montezemolo Nucetto Ormea Paroldo Perlo Priero Priola Prunetto Roascio Sale delle Langhe Sale San Giovanni Saliceto -

Sessione Per Eventi E Per Attività Sportive Autunno/Inverno
FONDAZIONE CRC SESSIONE PER EVENTI AUTUNNO INVERNO 2020 Settore Attività Istituzionale Sessione per Eventi e per Attività sportive autunno/inverno ESERCIZIO 2020 - INTERVENTI DELIBERATI CONSIGLIO DI AMMINISTRAZIONE DEL 14/09/2020 Ordinato per Settore - Località - Importo deliberato decrescente SVILUPPO LOCALE E INNOVAZIONE DA PAGINA 11 ARTE ATTIVITA' E BENI CULTURALI DA PAGINA 2 PROMOZIONE E SOLIDARIETA' SOCIALE DA PAGINA 10 EDUCAZIONE ISTRUZIONE E FORMAZIONE DA PAGINA 6 SALUTE PUBBLICA DA PAGINA 10 ATTIVITA' SPORTIVA DA PAGINA 7 1/15 FONDAZIONE CRC SESSIONE PER EVENTI AUTUNNO INVERNO 2020 Settore Attività Istituzionale IMPORTO SETTORE LOCALITA' ENTE RICHIEDENTE OGGETTO RICHIESTA DELIBERATO ASSOCIAZIONE 'ALEC - GIANFRANCO per la mostra fotografica 'Sotto la ARTE, ATTIVITA' E BENI CULTURALI ALBA - (CN) ALESSANDRIA' tenda di Abramo' 5.000,00 ASSOCIAZIONE CULTURALE per il progetto 'Scuola percussioni ARTE, ATTIVITA' E BENI CULTURALI ALBA - (CN) TIMBALES timbales' 3.000,00 per il progetto 'Aspettando Natale ARTE, ATTIVITA' E BENI CULTURALI ALBA - (CN) ASSOCIAZIONE CORALE INTONANDO 2020' 2.000,00 ASSOCIAZIONE CULTURALE per il progetto 'Burattinarte d'inverno ARTE, ATTIVITA' E BENI CULTURALI ALBA - (CN) BURATTINARTE 2021 - 22^ edizione' 2.000,00 per il progetto 'Jazz&co 8^ edizione: ARTE, ATTIVITA' E BENI CULTURALI ALBA - (CN) ASSOCIAZIONE MILLEUNANOTA linguaggi contemporanei' 2.000,00 per il progetto 'Narrar castelli e vini ARTE, ATTIVITA' E BENI CULTURALI ALBA - (CN) ASSOCIAZIONE TURISMO IN LANGA 2020' 2.000,00 ARTE, ATTIVITA' E BENI CULTURALI ALBA - (CN) IL NUCLEO per il progetto 'Il nucleo' 2.000,00 per 'Orizzonti festival - Sguardi oltre ARTE, ATTIVITA' E BENI CULTURALI ALBA - (CN) STRANI VARI ODV confine' 1.500,00 per il progetto 'Natale in San Giovanni - ARTE, ATTIVITA' E BENI CULTURALI ALBA - (CN) ASSOCIAZIONE SAN GIOVANNI ed. -

All a Plessi Sottodimensionati
Allegato A - Plessi in deroga A.S. 2020 - 2021 Piano dimensionamento rete scolastica - Plessi siti in Comuni marginali e montani da mantenere in deroga ENTE PROVINCIA DI CUNEO Allegato a Deliberazione Consiglio n. del DATI A.S. 2019/20 PUNTI DI EROGAZIONE DEL SERVIZIO DA MANTENERE IN DEROGA motivazione cod MIUR Comune sede cod MIUR Grado Comune sede (comune Istituto Tipo e denominazone Indirizzo istituto istituto plesso plesso Denominazione Indirizzo plesso plesso n. marginale/monta autonomo istituto autonomo autonomo autonomo scolastico scolastico plesso scolastico scolastico scolastico alunni no) montano/alternanza CNIC81000D I.C. di Robilante Piazza della Pace, 8 Robilante CNEE81005Q Primaria di Entracque Via A. Barale 2 Entracque 31 con Valdieri Piazza della Resistenza montano/alternanza CNIC81000D I.C. di Robilante Piazza della Pace, 8 Robilante CNEE81006R Primaria di Valdieri Valdieri 24 1 con Entracque CNIC80300A I.C. L. Romano Via Peano, 6 Demonte CNAA803039 Infanzia di Vinadio Via Trocello 10 Vinadio 15 montano CNIC80300A I.C. L. Romano Via Peano, 7 Demonte CNEE80305L Primaria di Vinadio/Aisone Via trocello, 12 Vinadio 31 montano Via Rocca de'Baldi, Secondaria n allievi in aumento dopo CNIC847004 I.C. Borgo San Giuseppe Cuneo CNMM847026 di Castelletto Stura Via Cuneo, 2 Castelletto Stura 24 15 di I grado accorp a Cuneo P.zza Battaglione montano CNIC82800P I.C. G. Giolitti Dronero CNEE828051 Primaria di Prazzo Via Nazionale 22 Prazzo 12 Alpini alta marginalità P.zza Battaglione S.Damiano CNIC82800P I.C. G. Giolitti Dronero CNEE828062 Primaria di S.Damiano Macra Via A. Beltricco 5 22 montano Alpini Macra P.zza Battaglione Secondaria CNIC82800P Giovanni Giolitti Dronero CNMM82802R di Stroppo Fraz. -

Disponibilità PERSONALE ATA 2021-2022
UFFICIO SCOLASTICO PROVINCIALE DI CUNEO POSTI DISPONIBILI PER SUPPLENZE COLLABORATORI SCOLASTICI Posto orario SEDE NOME 31-ago 1 POSTO ALBA CENTRO STORICO IC 31-ago 1 POSTO ALBA CTP 31-ago 1 POSTO ALBA CTP 31-ago 1 POSTO ALBA PIAVE SAN CASSIANO IC NAPOLITANO CARMINE 31-ago 1 POSTO ALBA PIAVE SAN CASSIANO IC 31-ago 1 POSTO ALBA PIAVE SAN CASSIANO IC 31-ago 1 POSTO ALBA PIAVE SAN CASSIANO IC 31-ago 1 POSTO ALBA PIAVE SAN CASSIANO IC 31-ago 1 POSTO ALBA QUARTIERE MORETTA IC SOFIA FEDERICA 31-ago 1 POSTO ALBA QUARTIERE MORETTA IC 31-ago 1 POSTO ALBA QUARTIERE MORETTA IC 31-ago 1 POSTO ALBA QUARTIERE MORETTA IC 31-ago 1 POSTO ALBA QUARTIERE MORETTA IC 31-ago 1 POSTO ALBA QUARTIERE MORETTA IC 31-ago 1 POSTO ALBA QUARTIERE MUSSOTTO IC 31-ago 1 POSTO ALBA QUARTIERE MUSSOTTO IC 31-ago 1 POSTO ALBA UMBERTO I IS CAPPATO DANIELE 31-ago 1 POSTO BENEVAGENNA IC 31-ago 1 POSTO BENEVAGENNA IC 31-ago 1 POSTO BENEVAGENNA IC 31-ago 1 POSTO BORGO SAN DALMAZZO IC 31-ago 1 POSTO BORGO SAN DALMAZZO IC 31-ago 1 POSTO BOSSOLASCO MURAZZANO IC NICOTRA SILVANO 31-ago 1 POSTO BOSSOLASCO MURAZZANO IC 31-ago 1 POSTO BOSSOLASCO MURAZZANO IC 31-ago 1 POSTO BOVES IC 31-ago 1 POSTO CANALE IC 31-ago 1 POSTO CANALE IC 31-ago 1 POSTO CARRU' IC 31-ago 1 POSTO CERVASCA IC 31-ago 1 POSTO CHERASCO IC 31-ago 1 POSTO CHERASCO IC 31-ago 1 POSTO CHIUSA PESIO PEVERAGNO IC 31-ago 1 POSTO CHIUSA PESIO PEVERAGNO IC 31-ago 1 POSTO CHIUSA PESIO PEVERAGNO IC 31-ago 1 POSTO CHIUSA PESIO PEVERAGNO IC 31-ago 1 POSTO CHIUSA PESIO PEVERAGNO IC 31-ago 1 POSTO CORTEMILIA SALICETO IC 31-ago 1 POSTO -

L'economia Cuneese
L’economia cuneese di fronte alla sfida della crisi 1 Per sopravvivere efficacemente in un ambiente competitivo la Provincia di Cuneo dovrà assumere la connotazione di Territorio sistema vitale Entità Entità spazialmente verso Socio-economica limitata organizzata GEO-COMUNITÀ Sviluppo Obiettivo: garantire la sopravvivenza Competitività 2 La provincia di Cuneo ricca di IMPRESE DI CRISTALLO Estremamente Estremamente preziose fragili Sono il motore Devono essere supportate, di sviluppo dell’economia difese e protette 3 Scenario di riferimento delle imprese di cristallo Tendenze per l’anno 2009 Diminuzione Mercati finanziari Azzeramento del potere Aree lontani della crescita New comers di acquisto di disagio sociale dalla ripresa del PIL reale Difficoltà di Contrazioni Rallentamento Redistribuzione Tensioni reperimento fondi dei consumi nello sviluppo dei mercati 4 La Provincia Granda di fronte alla sfida della recessione Scelte vincenti Basate su Sinergia Capacità Passione Capacità di cogliere Legame virtuosa di investire per il le richieste con il territorio tra i settori in mercati prodotto del mercato di nicchia Riflettono le VIRTÙ 5 La Granda nell’ambito del Piemonte sud-occidentale: la criticità CROCEVIA tra la nostra Penisola ed il resto dell’Europa Forte criticità per la Provincia di Cuneo Rischio di perdere il “treno” ⇓ Carenze infrastrutturali 83° posto per le infrastrutture Aeroporto Levaldigi Rete stradale Collegamento in cerca di soluzioni Rete ferroviaria Compromessa con la Francia efficaci per sviluppare obsoleta da una stasi inadeguato ai tempi inarrestabile le grandi potenzialità 6 Il diamante delle infrastrutture Stradale 250 Tot 200 Ferroviaria 150 100 Strutt. San Aereoporti e bacini 50 0 Strut. Istr. Energ. amb. Strut. Ricr Telefon. -

Medie Radon Provincia Cuneo 2017
Provincia Comune media radon al piano terra (Bq/m 3) Cuneo Acceglio 133 Cuneo Aisone 149 Cuneo Alba 99 Cuneo Albaretto della torre 79 Cuneo Alto 498 Cuneo Argentera 216 Cuneo Arguello 79 Cuneo Bagnasco 112 Cuneo Bagnolo Piemonte 135 Cuneo Baldissero d'Alba 105 Cuneo Barbaresco 89 Cuneo Barge 145 Cuneo Barolo 85 Cuneo Bastia mondovi' 108 Cuneo Battifollo 96 Cuneo Beinette 160 Cuneo Bellino 80 Cuneo Belvedere Langhe 79 Cuneo Bene Vagienna 148 Cuneo Benevello 79 Cuneo Bergolo 81 Cuneo Bernezzo 102 Cuneo Bonvicino 79 Cuneo Borgo San Dalmazzo 133 Cuneo Borgomale 79 Cuneo Bosia 87 Cuneo Bossolasco 79 Cuneo Boves 140 Cuneo Bra 146 Cuneo Briaglia 82 Cuneo Briga Alta 125 Cuneo Brondello 120 Cuneo Brossasco 118 Cuneo Busca 148 Cuneo Camerana 83 Cuneo Camo 80 Cuneo Canale 107 Cuneo Canosio 130 Cuneo Caprauna 602 Cuneo Caraglio 63 Cuneo Caramagna Piemonte 157 Cuneo Carde' 155 Cuneo Carru' 147 Cuneo Cartignano 116 Cuneo Casalgrasso 154 Cuneo Castagnito 92 Cuneo Casteldelfino 90 Cuneo Castellar 143 Cuneo Castelletto Stura 154 Cuneo Castelletto Uzzone 81 Cuneo Castellinaldo 98 Cuneo Castellino Tanaro 85 Cuneo Castelmagno 96 Cuneo Castelnuovo di Ceva 99 Cuneo Castiglione Falletto 94 Cuneo Castiglione Tinella 81 Cuneo Castino 81 Cuneo Cavallerleone 161 Cuneo Cavallermaggiore 160 Cuneo Celle di Macra 73 Cuneo Centallo 159 Cuneo Ceresole d'Alba 151 Cuneo Cerretto Langhe 79 Cuneo Cervasca 142 Cuneo Cervere 151 Cuneo Ceva 105 Cuneo Cherasco 140 Cuneo Chiusa di Pesio 147 Cuneo Ciglie' 98 Cuneo Cissone 79 Cuneo Clavesana 94 Cuneo Corneliano d'Alba 104 Cuneo -

Anno Scolastico 2018-19 PIEMONTE AMBITO 0020
Anno Scolastico 2018-19 PIEMONTE AMBITO 0020 - CN4 Elenco Scuole Primaria Ordinato sulla base della prossimità tra le sedi definita dall’ufficio territoriale competente SEDE DI ORGANICO ESPRIMIBILE DAL Altri Plessi Denominazione altri Indirizzo altri Comune altri PERSONALE Scuole stesso plessi-scuole stesso plessi-scuole stesso plessi-scuole Codice Istituto Denominazione Istituto DOCENTE Denominazione Sede Caratteristica Indirizzo Sede Comune Sede Istituto Istituto Istituto stesso Istituto CNIC855003 ALBA QUARTIERE CNEE855015 ALBA-BORGO MORETTA NORMALE VIA DE AMICIS N.1 ALBA MORETTA CNMM162004 CPIA 2 ALBA - MONDOVI' CNCT70000Q CENTRO TERRITORIALE ADULTI VIA DE AMICIS 1 ALBA ALBA CNCT703007 CENTRO TERRITORIALE ADULTI CORSO STATUTO, 17 MONDOVI' MONDOVI' CNIC854007 ALBA QUARTIERE PIAVE CNEE85402A ALBA-RODARI-PIAVE SAN NORMALE C.SO EUROPA,134 ALBA CNEE854019 ALBA - MARIA VIA SAN PIO V, 9 ALBA S.CASSIANO CASSIANO MONTESSORI CNIC85600V ALBA CENTRO STORICO CNEE856011 ALBA F.LLI NORMALE VIA F.LLI AMBROGIO ALBA AMBROGIO-CENTRO ST. CNIC84900Q DIANO CNEE849052 DIANO D'ALBA - CAP. NORMALE P.ZA UMBERTO I 25/A DIANO D'ALBA CNEE84902V GRINZANE CAVOUR - "S. VIA BRICCO 15 GRINZANE D'ALBA - CAPOLUOGO "EINAUDI" VIGLINO" CAVOUR CNEE849074 SINIO - "ELIGIO COCCIO" P.ZA DON MARCHISIO SINIO CNEE849063 RODELLO - CAPOLUOGO P.ZA COMUNALE, 4 RODELLO CNEE84901T ALBA - FRAZ. S.ROCCO SAN ROCCO CHERASCA ALBA CHERASCA N.2 CNEE84903X RODDI - "ELSA VIA SINEO, 1/A RODDI MALFERRARI" CNIC85300B ALBA QUARTIERE CNEE85301D ALBA-MUSSOTTO NORMALE VIA DELPIANO N. 11 ALBA CNEE85302E -
1. Asl 18 Alba-Bra Turni 2021 Aprile
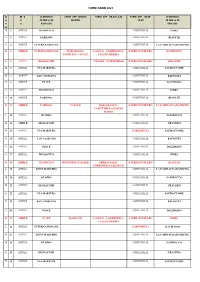
TURNI ANNO 2021 D M E DI SERVIZIO FARM. APP. ORARIO FARM. APP. 08,30-12,30 FARM. APP. 08,30- DI SERVIZIO A E 24 ORE su 24 DIURNO 19,30 24 ORE su 24 T S ALBA città BRA città A G 1 APRILE MUSSOTTO CORTEMILIA FIDES V 2 APRILE PARUSSO CORTEMILIA BIANCHI S 3 APRILE INTERNAZIONALE CORTEMILIA LA FARMACIA (BANDITO) D 4 APRILE INTERNAZIONALE MURAZZANO- CANALE - CORTEMILIA- S STEFANO BELBO SAN ROCCO NOVELLO- CANALE LEQUIO BERRIA L 5 APRILE DEGIACOMI CANALE - CORTEMILIA S STEFANO BELBO CRAVERO M 6 APRILE VIA MAESTRA CORTEMILIA SACRO CUORE M 7 APRILE SAN CASSIANO CORTEMILIA BAINOTTI G 8 APRILE PIAVE CORTEMILIA DALMASSO V 9 APRILE MUSSOTTO CORTEMILIA FIDES S 10 APRILE PARUSSO CORTEMILIA BIANCHI D 11 APRILE PARUSSO CANALE BOSSOLASCO - S STEFANO BELBO LA FARMACIA (BANDITO) CORTEMILIA-LEQUIO BERRIA L 12 APRILE DUOMO CORTEMILIA SAN ROCCO M 13 APRILE DEGIACOMI CORTEMILIA CRAVERO M 14 APRILE VIA MAESTRA CORTEMILIA SACRO CUORE G 15 APRILE SAN CASSIANO CORTEMILIA BAINOTTI V 16 APRILE PIAVE CORTEMILIA DALMASSO S 17 APRILE MUSSOTTO CORTEMILIA FIDES D 18 APRILE MUSSOTTO MONFORTE-CANALE SERRAVALLE- S STEFANO BELBO BIANCHI CORTEMILIA-LEQUIO B. L 19 APRILE DIVIN MAESTRO CORTEMILIA LA FARMACIA (BANDITO) M 20 APRILE DUOMO CORTEMILIA SAN ROCCO M 21 APRILE DEGIACOMI CORTEMILIA CRAVERO G 22 APRILE VIA MAESTRA CORTEMILIA SACRO CUORE V 23 APRILE SAN CASSIANO CORTEMILIA BAINOTTI S 24 APRILE PIAVE CORTEMILIA DALMASSO D 25 APRILE PIAVE DOGLIANI CANALE - CORTEMILIA- S STEFANO BELBO FIDES LEQUIO BERRIA L 26 APRILE INTERNAZIONALE CORTEMILIA DALMASSO M 27 APRILE DIVIN MAESTRO CORTEMILIA LA FARMACIA (BANDITO) M 28 APRILE DUOMO CORTEMILIA SAN ROCCO G 29 APRILE DEGIACOMI CORTEMILIA CRAVERO V 30 APRILE VIA MAESTRA CORTEMILIA SACRO CUORE TURNI ANNO 2021 D M E DI SERVIZIO FARM. -

Progetto Del Sistema Bibliotecario Monregalese Prov. Di Cuneo Referente Del Progetto : Livio Attanasio Tel. 0174 43003 E-Mail: [email protected]
Progetto del Sistema Bibliotecario Monregalese Prov. di Cuneo Referente del progetto : Livio Attanasio Tel. 0174 43003 e-mail: [email protected] Il progetto coinvolge i Comuni di: Comune coordinatore: Comune di Mondovì. Partecipano al progetto i seguenti Comuni: Magliano Alpi, Ceva, Montaldo Mondovì, Murazzano, Scagnello, Bagnasco, Frabosa Soprana, Farigliano, Camerana, Carrù, Garessio, Marsaglia, Monastero Vasco, Pianfei, Viola, Saliceto, Ormea, San Michele Mondovì, Villanova Mondovì, Belvedere Langhe, Lesegno, Bastia, Clavesana, Vicoforte, Sale Langhe, Dogliani, Piozzo, Mombasiglio, Roccaforte Mondovì, Castelnuovo di Ceva Scheda aggiornata al 30/06/2017 anno 2017-2018 Asili nido partecipanti al progetto: - Mondovì Asilo nido comunale a gestione diretta - Via Ortigara, 1 12084 Tel.0174-45157 e-mail: [email protected] Micronidi a gestione privata -Mondovì Micronido S. Anna – S.Anna Avagnina, Via dei Tigli, 12084 Tel. 0174-681744 e-mail: [email protected] -Mondovì Asilo nido “Lo gnomo scalzo”- Via Borgo Aragno, 1 12084 Tel. 0174-40830 e-mail: non disponibile -Mondovì Micronido “La capriola”-Via Rosa Bianca, 17 12084 Tel. 0174-481371 e-mail: [email protected] -Mondovì Baby parking “L’isola che non c’è”- Via del Christ, 29 12084 Tel. 0174-552773 e-mail: non disponibile -Mondovì Micronido “Zerotre”- C.so Statuto, 1 12084 Tel. 0174-554646 e-mail: [email protected] -Mondovì Micronido “Le Coccole”- Frazione Merlo, 12084 Cell. 3456246674 e-mail: [email protected] -Carrù Micronido “Il bosco incantato”- Via Garibaldi, 35 12061 Tel. 0173-759046 e-mail: [email protected] -Magliano Alpi Micronido “Stella Stellina”- Via Tomatis, 20 12060 Cell. 366 5661141/339 1277256 e-mail: [email protected] -Villanova Mondovì Micronido “Lo Scricciolo”- Via Don Rossi, 16 12089 Tel. -

Il Prefetto Della Provincia Di Cuneo
Il Prefetto della Provincia di Cuneo ALLEGATO A al Decreto prefettizio di convocazione dei comizi elettorali prot. n. 19149 del 25 marzo 2019 I Sig. Sindaci dei comuni interessati sono incaricati dell’esecuzione del presente decreto dandone notizia agli elettori con manifesto da affiggersi il quarantacinquesimo giorno antecedente a quello della convocazione. E’ da eleggere il numero di consiglieri indicato a fianco di ciascun Comune: Comune Abitanti al Censimento Comuni sino a 3000 abitanti 2011 Consiglieri da eleggere ALBARETTO DELLA TORRE 259 10 ARGUELLO 201 10 BAGNASCO 1038 10 BALDISSERO D'ALBA 1086 10 BAROLO 705 10 BATTIFOLLO 234 10 BELLINO 135 10 BELVEDERE LANGHE 370 10 BONVICINO 107 10 BORGOMALE 389 10 BOSIA 181 10 BOSSOLASCO 676 10 BRIAGLIA 324 10 BRIGA ALTA 48 10 BRONDELLO 287 10 CAMERANA 655 10 CANOSIO 82 10 CARDE' 1134 10 CARTIGNANO 178 10 CASTAGNITO 2113 10 CASTELLETTO STURA 1351 10 CASTELLETTO UZZONE 365 10 CASTELLINALDO D'ALBA 897 10 CASTELLINO TANARO 337 10 CASTIGLIONE FALLETTO 708 10 CASTINO 500 10 1 CAVALLERLEONE 652 10 CELLE DI MACRA 93 10 CERESOLE D'ALBA 2115 10 CERRETTO DELLE LANGHE 455 10 CERVERE 2162 10 CIGLIE' 196 10 CISSONE 82 10 CLAVESANA 900 10 CORNELIANO D'ALBA 2037 10 CRAVANZANA 408 10 DEMONTE 2059 10 ENVIE 2057 10 FARIGLIANO 1747 10 FAULE 496 10 FEISOGLIO 344 10 FRABOSA SOTTANA 1494 10 GAIOLA 600 10 GAMBASCA 408 10 GENOLA 2596 10 GORZEGNO 322 10 GOTTASECCA 174 10 GOVONE 2157 10 IGLIANO 84 10 ISASCA 78 10 LA MORRA 2718 10 LAGNASCO 1405 10 LEQUIO BERRIA 494 10 LESEGNO 854 10 LEVICE 246 10 LIMONE PIEMONTE 1490