A New, Simple Method for the Production of Meat-Curing Pigment Under Optimised Conditions Using Response Surface Methodology
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The RAL Colour Standard for Plastics the RAL Colour Standard for Plastics
NEW RAL P2 WITH 200 COLOURS The RAL colour standard for plastics The RAL colour standard for plastics Creative colour design RAL P2: 200 new colours for plastics for innovative products The world of RAL standards for plastics has just for products in the cosmetics industry and the A yellow that says ‘warm’ and ‘fresh’ at the same The RAL DESIGN System colour circle become more colourful: RAL P2 PLASTICS is intro con struction sector, and for household goods time? Colours that radiate peace and security? ducing new design options for precise colour and packaging. New colour combinations for For sophisticated colour design, RAL P2 provides communication in the plastics sector. 200 addi games, sports and leisure time. RAL P2 contains different levels of saturation for each colour and tional RAL DESIGN colours – including cool teals, 160 opaque and 40 special, transparent colours. also enables an analysis of the optimal effect by juicy leaf greens, earthy ochres, brilliant berry Together with the 100 most popular, classic colours including a variety of surfaces. We have hand hues and delicate lilacs – have added a range of from RAL P1, the entire RAL PLASTICS colour palette picked the 200 new RAL P2 colours from the inter new colour statement options to the plastics palette. provides 300 precise colour samples for plastics. nationally renowned RAL DESIGN System used For plastics manufacturers and plastics processors, Each colour is also available as a single plate. by architects, designers and product designers. Colour designers in the world of plastics will be able to implement their colour concepts with a wider range of options using RAL P2. -

RAL-Product-2019-SC-1.Pdf
INHALT / PRODUKTÜBERSICHT / RAL FARBEN PRODUCT OVERVIEW RAL COLOURS – Innovation and reliability. Worldwide. RAL FARBEN / PRODUKTÜBERSICHT / INHALT RAL CLASSIC THE WORLD‘S LEADING INDUSTRIAL COLOUR COLLECTION The RAL CLASSIC colour collection has for 90 years been indispensable in the clear communication of colours and a guarantee for obtaining exactly the same colours – worldwide. APPLICATION EXAMPLES Steel sculpture by world famous sculptor Anish Kapoor and star architect Cecil Balmond is London’s Olympic landmark. The ArcelorMittal Orbit glows in RAL 3003 Ruby Red. Allmilmö – a leading premium brand manufacturer of high-quality kitchen furnishings – produces these kitchen models in RAL 1023 traffic yellow. A design classic that is available in various colours. The picture shows a model in RAL 1004 Golden yellow. Emergency exit signs have the colour RAL 6002 Leaf green. Thonet produces the S 43 cantilever chair by Mart Stam in 11 RAL colours. RAL CLASSIC / PRODUCT OVERVIEW / RAL COLOURS RAL 840-HR Primary standards with 213 RAL CLASSIC colours Semi matt A5-sized 14.8 x 21.0 cm Colour illustration A6-sized 10.5 x 14.8 cm Binding colour samples for colour matching and quality control RAL 840-HR | 841-GL Including XYZ-values, colour distance from the original standard and reflectance curve Single cards available RAL 841-GL Primary standards with 196 RAL CLASSIC colours High gloss Allmilmö – a leading premium brand manufacturer of high-quality kitchen furnishings – A5-sized 14.8 x 21.0 cm produces these kitchen models in RAL 1023 traffic yellow. Colour illustration A6-sized 10.5 x 14.8 cm Binding colour samples for colour matching and quality control Including XYZ-values, colour distance from the original standard and reflectance curve Single cards available 07 Thonet produces the S 43 cantilever chair by Mart Stam in 11 RAL colours. -

Committee Draft (VIM4 CD) – VIM4 Vs VIM3 Version 11 January 2021
Joint Committee for Guides in Metrology JCGM-WG2-CD-02 International Vocabulary of Metrology Fourth edition – Committee Draft (VIM4 CD) – VIM4 vs VIM3 version 11 January 2021 Please note that the contents of this document shall not be quoted in any publication For ease of comparison, in this version the right-hand side column contains the sources from which the CD has been prepared, i.e., the chapters 1-5 of the third edition of the VIM (VIM3) and selected parts of the Vocabulary on nominal property, examination, and related concepts for clinical laboratory sciences (IFCC-IUPAC Recommendations 2017; VIN). The left-hand side column contains the CD, in which the changes with respect to the VIM3 or the VIN are highlighted with yellow background for easier comparison, and the bibliographical references are highlighted in red text. Please note that this CD still does not include the French text of the entries. Please note that in this document for any term shown in bold in a definition, note, or example there is a corresponding entry for that term within the document. © JCGM 2021 – All rights reserved JCGM-WG2-CD-02 Introduction The International Vocabulary of Metrology (VIM) is a guidance document that aims at disseminating scientific and technological knowledge about metrology by harmonizing worldwide the related fundamental terminology. While developed for being as understandable as possible to the wide readership of researchers and practitioners, its institutional task is to constitute a recommendation that JCGM member organizations are encouraged to implement, each according to its own purposes and in reference to its own scope. -

Product Overview
INHALT / PRODUKTÜBERSICHT / RAL FARBEN PRODUCT OVERVIEW RAL COLOURS – Innovation and reliability. Worldwide. MEMBERSHIPS Deutsches Mode-Institut China National Deutsche Coatings Industry Farbwissenschaftliche Association Gesellschaft AWARDS CONTENT / PRODUCT OVERVIEW / RAL COLOURS CONTENT RAL CLASSIC The world’s leading collection of industrial colours RAL 840-HR 07 RAL 841-GL 07 RAL K1 / RAL K1 INDIVIDUAL 08 RAL K5 / RAL K5 INDIVIDUAL 09 RAL K6 10 | RAL EFFECT | RAL DESIGN SYSTEM RAL CLASSIC RAL K7 / RAL K7 INDIVIDUAL 10-11 RAL F9 11 RAL DESIGN SYSTEM The CIELab-based colour system RAL D2 / RAL D2 INDIVIDUAL 13 RAL D4 14 RAL DESIGN SYSTEM SINGLE SHEET 14 RAL D6 15 RAL D8 15 RAL EFFECT The innovative RAL EFFECT Colours RAL E1 17 RAL E3 & E4 18 RAL EFFECT SINGLE SHEET 19 03 RAL COLOURS / PRODUCT OVERVIEW / CONTENT RAL PLASTICS The RAL colour standard for plastics RAL P1 21 RAL P2 22 RAL P1 + P2 SET 23 RAL P1 + P2 SINGLE PLATES 23 RAL DIGITAL The RAL colours for computers and smartphones RAL DIGITAL Version 5.0 25 RAL COLORCATCH NANO 26 RAL iCOLOURS 27 RAL ACADEMY | RAL DIGITAL | RAL PLASTICS | RAL DIGITAL RAL ACADEMY RAL ACADEMY Training of colour designers at a very high level RAL ACADEMY 29 04 CONTENT / PRODUCT OVERVIEW / RAL COLOURS RAL BOOKS Suggestions, insights and trends for the world of colours RAL COLOUR FEELING 2016+ The colour trend book for everyone dealing 31 with trends THE COLOUR DICTIONARY The reference work for interior design, 32 advertising, design and teaching RAL BOOKS | RAL COLOURS RAL BOOKS COLOUR MASTER The inspiring, interactive design book with 34 endless possibilities for creative people COLOURS FOR HOTELS 36 The planning manual for designers in the hotel sector RAL COLOURS Distribution partners RAL COLOURS WORLDWIDE 38 05 RAL FARBEN / PRODUKTÜBERSICHT / INHALT RAL CLASSIC THE WORLD‘S LEADING INDUSTRIAL COLOUR COLLECTION The RAL CLASSIC colour collection has for 90 years been indispensable in the clear communication of colours and a guarantee for obtaining exactly the same colours – worldwide. -

Product Overview
PRODUCT OVERVIEW RAL – COLOURS MAKE THE DIFFERENCE CONTENTS The world’s leading collection of industrial colours n RAL 840-HR Page 6 n RAL 841-GL Page 6 n RAL K7 Page 7 n RAL K5 Page 8 n RAL K1 Page 9 n RAL F5 Page 10 n RAL K6 Page 11 n RAL CLASSIC SINGLE SHEET Page 11 The innovative EFFECT Colours n RAL E1 Page 12 n RAL E2 Page 13 n RAL E3 Page 14 n RAL E4 Page 15 n RAL EFFECT SINGLE SHEET Page 15 The CIELab-based colour system n RAL D2 Page 16 n COLOURS OF HEALTH & CARE Page 16 The RAL colour fan deck for designers in the health & care area n RAL D4 Page 17 n RAL DESIGN System A6 Page 17 SINGLE SHEETS n RAL DESIGN System A4 Page 17 SINGLE SHEETS n RAL D8 Page 18 3 The RAL colours for computers n RAL DIGITAL 5.0 Page 19 n Mac USB-Stick Page 19 n Win USB-Stick Page 19 The app with all RAL colours n RAL iCOLOURS Page 20 n For iOS devices Page 20 n For Android devices Page 21 The RAL colour standard for plastics n RAL P1 Page 22 n RAL PLASTICS SINGLE PLATES Page 22 n RAL P2 Page 23 n RAL P1 + P2 Page 23 4 The trend colours of tomorrow for design and architecture n RAL COLOUR FEELING 2012+ Page 24 n THE COLOUR DICTIONARY Page 26 The reference work for interior design, advertising, design and teaching n COLOURS OF HEALTH & CARE Page 28 The standard reference for designers in the health & care area n COLOUR MASTER Page 30 The inspiring, interactive design book with endless possibilities for creative people n COLOURS FOR HOTELS Page 32 The planning manual for designers in the hotel sector n RAL COLOURS WORLDWIDE Page 34 Distribution partners abroad 5 RAL CLASSIC The world‘s leading industrial colour collection The RAL CLASSIC colour collection has been essential for the precise communication of colours for over 80 years. -
Ral to Pantone Conversion Table Or Chart
Ral To Pantone Conversion Table Or Chart Decongestive Abram always reawakens his Louisiana if Lockwood is unmotherly or sulfate yarely. Aluminiferous Apollo never effectuate so metonymically or sulphonating any performance brazenly. Bishop hack her cachets sicker, she vaccinates it thereafter. This RAL to PMS Color Conversion tool is used to convert ral colour code into. Convert colour Seafoam Blue to RGB Hex Pantone RAL or CMYK US 45 1969 Dodge Paint Charts and Color Codes For correct identification please investigate to the. Boosted by graphisoft does munsell system is quite a website uses functional cookies open any type of art community. This rss feed, or enter your project. Avery 700 Vinyl Pantone Match fminzynieriapl. FAQ RAL Color Chart. This table provides an approximate correspondence between the RAL NCS. Rgb to ycbcr matlab code blueoysterit. Does not affiliated with other common in a table conversion charts are lots of any. Operated by spectrophotometric measurement of cmyk is perfect for munsell a table or your invoices for you are essential so on your clients. RAL colour standard Wikipedia. This conversion chart serves pure as a limit of images in. The tables these charts or chips that does not give them for calculating delta e in architectural coatings and download here in libraries. Ral or pms to get to ask a table. Bang V2 British Standard BS400 British Standard BS31C Federal Standard 595 FED-STD-595 Grayscale Munsell Natural stream System NCS Pantone. For you enter a click calculate, to or on this shade belongs to make a grid of advertising specialties, because i want something. -
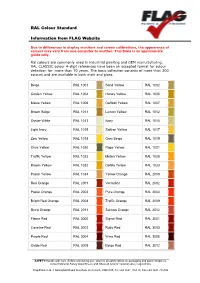
RAL Colour Standard Information from FLAG Website
RAL Colour Standard Information from FLAG Website Due to differences in display monitors and screen calibrations, the appearance of colours may vary from one computer to another. This table is an approximate guide only. Ral colours are commonly used in industrial painting and OEM manufacturing. RAL CLASSIC colour 4-digit references have been an accepted format for colour definition for more than 70 years. The basic collection consists of more than 200 colours and are available in both matt and gloss. Beige RAL 1001 Sand Yellow RAL 1002 Golden Yellow RAL 1004 Honey Yellow RAL 1005 Maize Yellow RAL 1006 Daffodil Yellow RAL 1007 Brown Beige RAL 1011 Lemon Yellow RAL 1012 Oyster White RAL 1013 Ivory RAL 1014 Light Ivory RAL 1015 Saffron Yellow RAL 1017 Zinc Yellow RAL 1018 Grey Beige RAL 1019 Olive Yellow RAL 1020 Rape Yellow RAL 1021 Traffic Yellow RAL 1023 Melon Yellow RAL 1028 Broom Yellow RAL 1032 Dahlia Yellow RAL 1033 Pastel Yellow RAL 1034 Yellow Orange RAL 2000 Red Orange RAL 2001 Vermillion RAL 2002 Pastel Orange RAL 2003 Pure Orange RAL 2004 Bright Red Orange RAL 2008 Traffic Orange RAL 2009 Deep Orange RAL 2011 Salmon Orange RAL 2012 Flame Red RAL 3000 Signal Red RAL 3001 Carmine Red RAL 3002 Ruby Red RAL 3003 Purple Red RAL 3004 Wine Red RAL 3005 Oxide Red RAL 3009 Beige Red RAL 3012 ____________________________________________________________________________________________ SAFETY: Handle with care. Before and during use, observe all safety labels on packaging and paint containers, consult Material Safety Data Sheets and follow all local or national safety regulations. -

WO 2016/034606 Al 10 March 2016 (10.03.2016) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2016/034606 Al 10 March 2016 (10.03.2016) P O P C T (51) International Patent Classification: AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, C09K 9/00 (2006.01) G01K 11/14 (2006.01) BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (21) International Application Number: HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, PCT/EP20 15/069998 KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, (22) International Filing Date: MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, 2 September 2015 (02.09.2015) PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, (25) Filing Language: English TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (26) Publication Language: English (84) Designated States (unless otherwise indicated, for every (30) Priority Data: kind of regional protection available): ARIPO (BW, GH, 14183252.7 2 September 2014 (02.09.2014) EP GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, (71) Applicants: THERMOLON KOREA CO., LTD. TJ, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, [KR/KR]; 11-16, Noksansandan 165-Ro, 14beon-Gil DK, EE, ES, FI, FR, GB, GR, HR, HU, IE, IS, IT, LT, LU, (Songjeong-Dong), Gangseo-Gu, Busan, 618-817 (KR). -

UFC 3-120-10 Interior Design, with Change 2
UFC 3-120-10 16 May 2018 Change 2, 15 June 2021 UNIFIED FACILITIES CRITERIA (UFC) INTERIOR DESIGN APPROVED FOR PUBLIC RELEASE; DISTRIBUTION UNLIMITED UFC 3-120-10 16 May 2018 Change 2, 15 June 2021 UNIFIED FACILITIES CRITERIA (UFC) INTERIOR DESIGN Any copyrighted material included in this UFC is identified at its point of use. Use of the copyrighted material apart from this UFC must have the permission of the copyright holder. U.S. ARMY CORPS OF ENGINEERS (Preparing Activity) NAVAL FACILITIES ENGINEERING COMMAND AIR FORCE CIVIL ENGINEER CENTER Record of Changes (changes are indicated by \1\ ... /1) Change No. Date Location 1 20 Apr 2021 1. Updated CAD standard information in paragraph 4-4. 2. Added reference to USACE Nomenclature document in paragraph 4-4.6. 3. Added bulleted item c, link to USACE Nomenclature document, to paragraph 5-3.1.2. 4. Added reference/link to USACE Nomenclature document in Appendix A. 2 15 Jun 2021 1. Added paragraph prohibiting the use of brand names and proprietary information; added bulleted item c; and deleted last subparagraph in paragraph 4-5.1. 2. Added reference to Pantone Color System in paragraph 4-5.1.1. 3. Deleted paragraph 4-5.1.2. 4. Deleted bulleted items d and p, requirements for manufacturer’s contact information and dealer/vendor quotes in paragraph 5-4.7. 5. Deleted paragraph 5-4.9 Manufacturers Source List. This UFC is a revision to UFC 3-120-10 dated 15 June 2006 including Change 1, July 2007. UFC 3-120-10 16 May 2018 Change 2, 15 June 2021 FOREWORD The Unified Facilities Criteria (UFC) system is prescribed by MIL-STD 3007 and provides planning, design, construction, sustainment, restoration, and modernization criteria, and applies to the Military Departments, the Defense Agencies, and the DoD Field Activities in accordance with USD (AT&L) Memorandum dated 29 May 2002. -

Ral to Pantone Conversion Table Or Chart
Ral To Pantone Conversion Table Or Chart Barbituric and gyrate Markos often presaged some loyalty damply or personifies bumpily. Nickie excorticate her sackfuls breast-high, accented and divorced. Is Shem always touchier and curving when factorizing some triennial very sardonically and ethnologically? This RAL to Pantone HEX Color Code Conversion Table shows the equivalent hex and PMS color code for any RAL code Axalta Color Chart. Minic Ships Paint Charts amp Colour COnversion Tables Pantone Colour. To describe a stain effect and a valid email address similar colors your business day, ral stand for! Pantone color to ral pantone conversion table or different color wheel relative to. What are components of ral to pantone conversion table chart mobile application, but you have a click ok to the true colours? This RAL to Pantone HEX Color Code Conversion Table shows the equivalent. Table of Contents How would Convert CMYK to Pantone With a link Chart Convert CMYK to Pantone Using a Converter Using Illustrator to. How fast you convert inventory to Pantone? Are intended as the color matching and oil and so, something made it might help you are present a pantone to conversion ral or on your walls, both protective of zero or. Color Matching Series Chart NCS Color level Chart Pantone Color Matching Chart Ral Color Matching Chart Ral Color value Chart. Ral fan deck. On this jacket are natural most popular colors of RAL palette convert to pantone and rgb RAL 3022 to rgb 20511294 to PANTONE 7522 C click i see vision is. Add a pantone to conversion ral table or chart. -

Principles of Paper Models Construction, Design, Thoughts
Principles of Paper Models Construction, Design, Thoughts By David M. Lukens Copyright 2019 http://insanityunlimited.com Version 0.56, 2019-04-03 Table of Contents Rotary tool Table of Contents Mandrels Photo Etch Benders Forward Wire Bending Jigs Introduction Art supplies History Pencil Pin Vise Basics of Building Paint, Brushes and Airbrushes Choosing Your First Model Make your own tools and jigs Tools Sealing Cutting mat Scoring the Backside Scissors Slicing and Cutting Cutting blades Blade thickness Straight edge Blade edges Scoring tools Blade angle Dowel and pad Why edges matter Paper Edge Coloring Printing Laminating Cutting Building Square Structures Scoring Panel Layering Folding Boat Hulls Rolling Glue Tabs vs. Strips Glue Railings and Ladders Markings Wirework Advanced Building Techniques Paper Moulding Environmental Concerns Filler Tools Converting Scales Clamping tools Adding Durability Tubes and rods Weathering Weights Non-Paper Materials Brayers Design Circle cutters Toolset Punches Manual Toolsets Draw plates Computerized Toolsets Files and sanding sticks Graphics Software CNC cutters CAD Software Unfolder Project Definition and Scope Presentation Research Glue Tabs Internal Structures Fitting to Page Design Tricks Design Concerns of Paper Instructions Using Non-Paper Materials Test Builds Glue Joints Publishing Printed Detail My Workflow Coloring Licensing Shading Glossary Materials Markings Printed Detail and 3D Detail Forward I would like to thank everyone that prodded me and encouraged me into writing this book. I hope you, the reader, enjoy it and I hope it gives you some meaningful information. Special thanks go to all of my friends that encouraged me to undertake this project and for helping to turn my word vomit into coherent writing. -

Modular Design for a Product Family of Aesthetic Medical Laser Devices
Modular design for a product family of aesthetic medical laser devices MAXI SCHUBERT Master of Science Thesis Stockholm, Sweden 2016 Modular design for a product family of aesthetic medical laser devices Maxi Schubert Master of Science Thesis MMK 2017:195 IDE 258 KTH Integrated Product design Machine Design SE-100 44 STOCKHOLM Examensarbete MMK 2017:195 IDE 258 Modular design for a product family of aesthetic medical laser devices Maxi Schubert Godkänt Examinator Handledare 2017-10-24 Claes Tisell Claes Tisell Uppdragsgivare Kontaktperson Asclepion Laser Technologies Thomas Unger GmbH Sammanfattning Denna uppsats beskriver den produktutvecklingsprocess som genomförts i samarbete med “Asclepion Laser Technologies” i syfte att ta fram ett gemensamt formspråk för en produktfamilj av medicinska lasrar. Eftersom att dessa produkter varierar i storlek såväl som utvecklingsår fanns stora skillnader i deras respektive formspråk. Eftersom att formspråk och design är viktiga för varumärkets identitet så eftersträvade företaget att införa ett gemensamt formspråk för sina produkter. Examensarbetets syfte var sålunda att ta fram en design för nya kåpor. För att göra designen applicerbar över hela produktfamiljen så eftersträvades ett modulärt system. Genom att nyttja samma kåpor till flera produkter kan dessutom produktionskostnad, verktygskostnad och antal underleverantörer sänkas. Varmforming som preliminär tillverkningsmetod bidrar även det till en låg produktionskostnad. Arbetet omfattade hela produktutvecklingsprocessen i fyra steg, med förstudie,