Oxycontin (Oxycodone)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Vaccine Adverse Event Reporting System (VAERS)
"Notes" "Symptoms" "Symptoms Code" Events Reported Percent "17-HYDROXYPROGESTERONE INCREASED" "10063263" 1 0.00% "5'NUCLEOTIDASE INCREASED" "10000028" 1 0.00% "ABASIA" "10049460" 1309 0.20% "ABDOMEN SCAN" "10061936" 10 0.00% "ABDOMEN SCAN NORMAL" "10061937" 13 0.00% "ABDOMINAL COMPARTMENT SYNDROME" "10058808" 2 0.00% "ABDOMINAL DISCOMFORT" "10000059" 1930 0.30% "ABDOMINAL DISTENSION" "10000060" 505 0.08% "ABDOMINAL EXPLORATION" "10053309" 1 0.00% "ABDOMINAL HERNIA" "10060954" 1 0.00% "ABDOMINAL HERNIA REPAIR" "10060802" 2 0.00% "ABDOMINAL INFECTION" "10056519" 3 0.00% "ABDOMINAL INJURY" "10060924" 3 0.00% "ABDOMINAL LYMPHADENOPATHY" "10073485" 3 0.00% "ABDOMINAL MASS" "10000077" 21 0.00% "ABDOMINAL OPERATION" "10061609" 6 0.00% "ABDOMINAL PAIN" "10000081" 5426 0.84% "ABDOMINAL PAIN LOWER" "10000084" 171 0.03% "ABDOMINAL PAIN UPPER" "10000087" 3699 0.57% "ABDOMINAL RIGIDITY" "10000090" 32 0.00% "ABDOMINAL SYMPTOM" "10060926" 3 0.00% "ABDOMINAL TENDERNESS" "10000097" 62 0.01% "ABDOMINAL WALL ABSCESS" "10000099" 1 0.00% "ABDOMINAL WALL DISORDER" "10059625" 1 0.00% "ABDOMINAL WALL NEOPLASM MALIGNANT" "10071023" 1 0.00% "ABDOMINAL X-RAY" "10061612" 279 0.04% "ABNORMAL BEHAVIOUR" "10061422" 1775 0.27% "ABNORMAL CLOTTING FACTOR" "10049862" 1 0.00% "ABNORMAL DREAMS" "10000125" 133 0.02% "ABNORMAL FAECES" "10000133" 247 0.04% "ABNORMAL LABOUR" "10000153" 37 0.01% "ABNORMAL LABOUR AFFECTING FOETUS" "10000154" 1 0.00% "ABNORMAL LOSS OF WEIGHT" "10000159" 23 0.00% "ABNORMAL PRODUCT OF CONCEPTION" "10060927" 2 0.00% "ABNORMAL SENSATION IN EYE" "10000173" -

ICD-9 Diagnosis Codes Effective 10/1/2011 (V29.0) Source: Centers for Medicare and Medicaid Services
ICD-9 Diagnosis Codes effective 10/1/2011 (v29.0) Source: Centers for Medicare and Medicaid Services 0010 Cholera d/t vib cholerae 00801 Int inf e coli entrpath 01086 Prim prg TB NEC-oth test 0011 Cholera d/t vib el tor 00802 Int inf e coli entrtoxgn 01090 Primary TB NOS-unspec 0019 Cholera NOS 00803 Int inf e coli entrnvsv 01091 Primary TB NOS-no exam 0020 Typhoid fever 00804 Int inf e coli entrhmrg 01092 Primary TB NOS-exam unkn 0021 Paratyphoid fever a 00809 Int inf e coli spcf NEC 01093 Primary TB NOS-micro dx 0022 Paratyphoid fever b 0081 Arizona enteritis 01094 Primary TB NOS-cult dx 0023 Paratyphoid fever c 0082 Aerobacter enteritis 01095 Primary TB NOS-histo dx 0029 Paratyphoid fever NOS 0083 Proteus enteritis 01096 Primary TB NOS-oth test 0030 Salmonella enteritis 00841 Staphylococc enteritis 01100 TB lung infiltr-unspec 0031 Salmonella septicemia 00842 Pseudomonas enteritis 01101 TB lung infiltr-no exam 00320 Local salmonella inf NOS 00843 Int infec campylobacter 01102 TB lung infiltr-exm unkn 00321 Salmonella meningitis 00844 Int inf yrsnia entrcltca 01103 TB lung infiltr-micro dx 00322 Salmonella pneumonia 00845 Int inf clstrdium dfcile 01104 TB lung infiltr-cult dx 00323 Salmonella arthritis 00846 Intes infec oth anerobes 01105 TB lung infiltr-histo dx 00324 Salmonella osteomyelitis 00847 Int inf oth grm neg bctr 01106 TB lung infiltr-oth test 00329 Local salmonella inf NEC 00849 Bacterial enteritis NEC 01110 TB lung nodular-unspec 0038 Salmonella infection NEC 0085 Bacterial enteritis NOS 01111 TB lung nodular-no exam 0039 -

Actiq (Oral Transmucosal Fentanyl)
Texas Prior Authorization Program Clinical Edit Criteria Drug/Drug Class Actiq (Oral Transmucosal Fentanyl) Clinical Edit Information Included in this Document Actiq 200mcg • Drugs requiring prior authorization: the list of drugs requiring prior authorization for this clinical edit • Prior authorization criteria logic: a description of how the prior authorization request will be evaluated against the clinical edit criteria rules • Logic diagram: a visual depiction of the clinical edit criteria logic • Supporting tables: a collection of information associated with the steps within the criteria (diagnosis codes, procedure codes, and therapy codes); provided when applicable • References: clinical publications and sources relevant to this clinical edit Actiq Greater Than or Equal To (≥) 400mcg • Drugs requiring prior authorization: the list of drugs requiring prior authorization for this clinical edit • Prior authorization criteria logic: a description of how the prior authorization request will be evaluated against the clinical edit criteria rules • Logic diagram: a visual depiction of the clinical edit criteria logic • Supporting tables: a collection of information associated with the steps within the criteria (diagnosis codes, procedure codes, and therapy codes); provided when applicable • References: clinical publications and sources relevant to this clinical edit Note: Click the hyperlink to navigate directly to that section. February 7, 2012 Copyright © 2011-2012 Health Information Designs, LLC 1 Texas Prior Authorization Program Clinical -

The 12 Cranial Nerves
The 12 Cranial Nerves Edited by Sterling Precision Nelson Cranial Nerve I: Olfactory Nerve Type of Nerve: Sensory Function of Nerve: Sense of Smell Brief description: The Olfactory Nerve is the first of the 12 pairs of cranial nerves in the brain. It’s a sensory nerve, in charge of transmitting olfactory stimuli from the nose to the brain, Its actual origin is given by the cells of the olfactory bulb and is the shortest cranial pair of all of the pairs in the brain. Important side note: Damaged olfactory nerve cells can regenerate, but don’t always reconnect properly in the brain. Name Nerve Disorder Treatment Dysnomia - Parosmia Distortion of smelling that No specific cure refers to the perception of available, but symptoms the smell. lessen over time, in some patients it may take years. Dysnomia - Phantosmia Distortion of smelling that Can recover easily if it refers to a smell not from sickness however present. rinsing out the sinus with saline solution will help treat the problem. Hyposmia A decreased ability to there's no known cure or smell. treatment for congenital anosmia. Anosmia A total inability to smell. there's no known cure or treatment for congenital anosmia. Cranial Nerve II: Optical Type of Nerve: Sensory Function of Nerve: Vision Brief description: The Optical cranial pair is the second of the 12 pairs of cranial nerves and it is responsible for conducting visual stimuli from the eye to the brain. It is made of axons from the ganglion cells of the retina, that take the information of the photoreceptors to the brain, where later it will be integrated and interpreted and It emerges in the diencephalon. -

Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0 Published: November 27, 2017
Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0 Published: November 27, 2017 U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Common Terminology Criteria for Adverse Events (CTCAE) v5.0 Publish Date: November 27, 2017 Introduction Grades Grade 5 The NCI Common Terminology Criteria for Grade refers to the severity of the AE. The CTCAE Grade 5 (Death) is not appropriate for some AEs Adverse Events is a descriptive terminology which displays Grades 1 through 5 with unique clinical and therefore is not an option. can be utilized for Adverse Event (AE) reporting. A descriptions of severity for each AE based on this grading (severity) scale is provided for each AE general guideline: Definitions term. A brief Definition is provided to clarify the Grade 1 Mild; asymptomatic or mild meaning of each AE term. A single dash (-) symptoms; clinical or diagnostic indicates a Definition is not available. SOC observations only; intervention System Organ Class (SOC), the highest level of the not indicated. 1 MedDRA hierarchy, is identified by anatomical or Grade 2 Moderate; minimal, local or Navigational Notes physiological system, etiology, or purpose (e.g., noninvasive intervention A Navigational Note is used to assist the reporter SOC Investigations for laboratory test results). indicated; limiting age- in choosing a correct AE. It may list other AEs that CTCAE terms are grouped by MedDRA Primary appropriate instrumental ADL*. should be considered in addition to or in place of SOCs. Within each SOC, AEs are listed and Grade 3 Severe or medically significant but not the AE in question. A single dash (-) indicates a accompanied by descriptions of severity (Grade). -
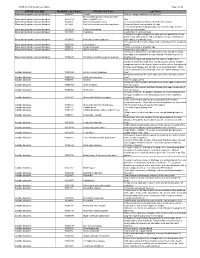
CTCAE V4.0 and Lay Term Mapping Document
CTCAE v4.0 Terms with Lay Terms Page 1 of 15 CTCAE v4.0 SOC MedDRA v12.0 Code CTCAE v4.0 Term Lay Term Blood and lymphatic system disorders 10002272 Anemia Lack of enough red blood cells (anemia) Blood and lymphatic system disorders - Blood and lymphatic system disorders 10005329 Other, [add AE here] N/A Blood and lymphatic system disorders 10048580 Bone marrow hypocellular Decreased number of blood cells in the bone marrow Blood and lymphatic system disorders 10013442 Disseminated intravascular coagulation Abnormal blood clotting and/or bleeding Fever associated with dangerously low levels of a type of white Blood and lymphatic system disorders 10016288 Febrile neutropenia blood cell (neutrophils) Blood and lymphatic system disorders 10019491 Hemolysis Destruction of red blood cells Collection of signs including hemolytic anemia (destruction of red blood cells), kidney failure and a low platelet (a type of blood cell Blood and lymphatic system disorders 10019515 Hemolytic uremic syndrome that helps to clot blood) count Increased number of white blood cells (leukocytes) in the peripheral Blood and lymphatic system disorders 10024378 Leukocytosis blood Blood and lymphatic system disorders 10025182 Lymph node pain Painful sensation in a lymph node Blood and lymphatic system disorders 10041633 Spleen disorder Abnormality of the spleen Formation of blood clots in small blood vessels around the body that leads to a low platelet (a type of blood cell that helps to clot Blood and lymphatic system disorders 10043648 Thrombotic thrombocytopenic purpura blood) count Collection of signs and symptoms that indicate sudden heart disease in which the heart does not get enough oxygen. -

ICD-9 Diagnosis Codes Effective 10/1/2006 (V24.0) Source: Centers for Medicare and Medicaid Services
ICD-9 Diagnosis Codes effective 10/1/2006 (v24.0) Source: Centers for Medicare and Medicaid Services 0010 CHOLERA D/T VIB CHOLERAE 0082 AEROBACTER ENTERITIS 01104 TB LUNG INFILTR-CULT DX 0011 CHOLERA D/T VIB EL TOR 0083 PROTEUS ENTERITIS 01105 TB LUNG INFILTR-HISTO DX 0019 CHOLERA NOS 00841 STAPHYLOCOCC ENTERITIS 01106 TB LUNG INFILTR-OTH TEST 0020 TYPHOID FEVER 00842 PSEUDOMONAS ENTERITIS 01110 TB LUNG NODULAR-UNSPEC 0021 PARATYPHOID FEVER A 00843 INT INFEC CAMPYLOBACTER 01111 TB LUNG NODULAR-NO EXAM 0022 PARATYPHOID FEVER B 00844 INT INF YRSNIA ENTRCLTCA 01112 TB LUNG NODUL-EXAM UNKN 0023 PARATYPHOID FEVER C 00845 INT INF CLSTRDIUM DFCILE 01113 TB LUNG NODULAR-MICRO DX 0029 PARATYPHOID FEVER NOS 00846 INTES INFEC OTH ANEROBES 01114 TB LUNG NODULAR-CULT DX 0030 SALMONELLA ENTERITIS 00847 INT INF OTH GRM NEG BCTR 01115 TB LUNG NODULAR-HISTO DX 0031 SALMONELLA SEPTICEMIA 00849 BACTERIAL ENTERITIS NEC 01116 TB LUNG NODULAR-OTH TEST 00320 LOCAL SALMONELLA INF NOS 0085 BACTERIAL ENTERITIS NOS 01120 TB LUNG W CAVITY-UNSPEC 00321 SALMONELLA MENINGITIS 00861 INTES INFEC ROTAVIRUS 01121 TB LUNG W CAVITY-NO EXAM 00322 SALMONELLA PNEUMONIA 00862 INTES INFEC ADENOVIRUS 01122 TB LUNG CAVITY-EXAM UNKN 00323 SALMONELLA ARTHRITIS 00863 INT INF NORWALK VIRUS 01123 TB LUNG W CAVIT-MICRO DX 00324 SALMONELLA OSTEOMYELITIS 00864 INT INF OTH SML RND VRUS 01124 TB LUNG W CAVITY-CULT DX 00329 LOCAL SALMONELLA INF NEC 00865 INTES INFEC CALCIVIRUS 01125 TB LUNG W CAVIT-HISTO DX 0038 SALMONELLA INFECTION NEC 00866 INTES INFEC ASTROVIRUS 01126 TB LUNG W CAVIT-OTH -

32Nd European Neurology Congress
conferenceseries.com 32nd European Neurology Congress July 22-24, 2019 London, UK SCIENTIFIC PROGRAM SCIENTIFIC PROGRAM Monday, 22nd July DAY 1 Monday, 22nd July 08:30-09:00 Registrations 09:00-09:30 Introduction 09:30-09:50 COFFEE BREAK 09:50-11:50 KEYNOTE LECTURES Meeting Hall 01 MEETING HALL 01 MEETING HALL 02 11:50-13:10 Talks On: Neurology Talks On: Clinical Neurophysiology Central nervous system Electromyography Peripheral nervous system Electroencephalography Cerebrovascular disease Evoked potentials Behavioral neurology Intraoperative monitoring 13:10-13:15 GROUP PHOTO 13:15-14:00 LUNCH BREAK MEETING HALL 01 MEETING HALL 02 14:00-16:00 Talks On: Neurosurgery Talks On: Central Nervous System Vascular neurosurgery Bipolar disorder Stereotactic neurosurgery/ functional Neuropathic pain syndromes neurosurgery Oncological neurosurgery Accessory nerve disorder Skull base surgery CNS disorder and structural defects Spinal neurosurgery Facial nerve paralysis Pediatric neurosurgery Meningitis 16:00-16:20 COFFEE BREAK MEETING HALL 01 (16:20-17:00) MEETING HALL 01 (17:00-18:00) Young Researchers in Neurology Workshop Visit: https://www.neurologyconference.com/europe/ SCIENTIFIC PROGRAM Tuesday, 23rd July DAY 2 Tuesday, 23rd July 09:00-10:30 KEYNOTE LECTURES Meeting Hall 01 10:30-10:50 COFFEE BREAK MEETING HALL 01 MEETING HALL 02 10:50-12:50 Talks On: Pediatric Neurology Talks On: Neuromuscular Disorders Movement disorders (Cerebral paresis) Amyotrophic lateral sclerosis Muscle diseases Multiple sclerosis Lysosomal storage disease Myasthenia -

Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0 Published: May 28, 2009 (V4.03: June 14, 2010)
1. CTCAE 4.03 Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0 Published: May 28, 2009 (v4.03: June 14, 2010) U.S.DEPARTMENT OF HEALTH AND HUMAN SERVICES National Institutes of Health National Cancer Institute Common Terminology Criteria for Adverse Events v4.0 (CTCAE) Publish Date: May 28, 2009 Quick Reference Definitions Not all Grades are appropriate for all AEs. The NCI Common Terminology Criteria for A brief definition is provided to clarify the Therefore, some AEs are listed with fewer than Adverse Events is a descriptive terminology which meaning of each AE term. five options for Grade selection. can be utilized for Adverse Event (AE) reporting. Grade 5 A grading (severity) scale is provided for each AE Grades term. Grade refers to the severity of the AE. The CTCAE Grade 5 (Death) is not appropriate for some AEs displays Grades 1 through 5 with unique clinical and therefore is not an option. Components and Organization descriptions of severity for each AE based on this Activities of Daily Living (ADL) SOC general guideline: *Instrumental ADL refer to preparing meals, System Organ Class, the highest level of the Grade 1 Mild; asymptomatic or mild shopping for groceries or clothes, using the MedDRA hierarchy, is identified by anatomical or symptoms; clinical or diagnostic telephone, managing money, etc. physiological system, etiology, or purpose (e.g., observations only; intervention not SOC Investigations for laboratory test results). indicated. **Self care ADL refer to bathing, dressing and CTCAE terms are grouped by MedDRA Primary Grade 2 Moderate; minimal, local or undressing, feeding self, using the toilet, taking SOCs. -

Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0 Published: November 27, 2017
Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0 Published: November 27, 2017 U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES National Institutes of Health National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) v5.0 Publish Date: November 27, 2017 Introduction Grades Grade 5 The NCI Common Terminology Criteria for Adverse Grade refers to the severity of the AE. The CTCAE Grade 5 (Death) is not appropriate for some AEs Events is a descriptive terminology which can be displays Grades 1 through 5 with unique clinical and therefore is not an option. utilized for Adverse Event (AE) reporting. A grading descriptions of severity for each AE based on this (severity) scale is provided for each AE term. general guideline: Definitions A brief Definition is provided to clarify the meaning Grade 1 Mild; asymptomatic or mild symptoms; SOC of each AE term. A single dash (-) indicates a clinical or diagnostic observations only; System Organ Class (SOC), the highest level of the Definition is not available. intervention not indicated. MedDRA1 hierarchy, is identified by anatomical or Grade 2 Moderate; minimal, local or noninvasive physiological system, etiology, or purpose (e.g., Navigational Notes intervention indicated; limiting age- SOC Investigations for laboratory test results). A Navigational Note is used to assist the reporter in appropriate instrumental ADL*. CTCAE terms are grouped by MedDRA Primary choosing a correct AE. It may list other AEs that Grade 3 Severe or medically significant but not SOCs. Within each SOC, AEs are listed and should be considered in addition to or in place of immediately life-threatening; accompanied by descriptions of severity (Grade). -

Oxycodone Extended-Release Agents
Texas Prior Authorization Program Clinical Edit Criteria Drug/Drug Class Oxycodone Extended-Release Agents Clinical Edit Information Included in this Document Oxycodone ER - Low Dose Drugs requiring prior authorization: the list of drugs requiring prior authorization for this clinical edit Prior authorization criteria logic: a description of how the prior authorization request will be evaluated against the clinical edit criteria rules Logic diagram: a visual depiction of the clinical edit criteria logic Supporting tables: a collection of information associated with the steps within the criteria (diagnosis codes, procedure codes, and therapy codes); provided when applicable References: clinical publications and sources relevant to this clinical edit Oxycodone ER - High Dose Drugs requiring prior authorization: the list of drugs requiring prior authorization for this clinical edit Prior authorization criteria logic: a description of how the prior authorization request will be evaluated against the clinical edit criteria rules Logic diagram: a visual depiction of the clinical edit criteria logic Supporting tables: a collection of information associated with the steps within the criteria (diagnosis codes, procedure codes, and therapy codes); provided when applicable References: clinical publications and sources relevant to this clinical edit Note: Click the hyperlink to navigate directly to that section. November 20, 2017 Copyright © 2011-2017 Health Information Designs, LLC 1 Texas Prior Authorization Program Clinical Edits Oxycodone -

Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0 Published: May 28, 2009 (V4.03: June 14, 2010)
1. CTCAE 4.03 Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0 Published: May 28, 2009 (v4.03: June 14, 2010) U.S.DEPARTMENT OF HEALTH AND HUMAN SERVICES National Institutes of Health National Cancer Institute Common Terminology Criteria for Adverse Events v4.0 (CTCAE) Publish Date: May 28, 2009 Quick Reference Definitions Not all Grades are appropriate for all AEs. The NCI Common Terminology Criteria for A brief definition is provided to clarify the Therefore, some AEs are listed with fewer than Adverse Events is a descriptive terminology which meaning of each AE term. five options for Grade selection. can be utilized for Adverse Event (AE) reporting. Grade 5 A grading (severity) scale is provided for each AE Grades term. Grade refers to the severity of the AE. The CTCAE Grade 5 (Death) is not appropriate for some AEs displays Grades 1 through 5 with unique clinical and therefore is not an option. Components and Organization descriptions of severity for each AE based on this Activities of Daily Living (ADL) SOC general guideline: *Instrumental ADL refer to preparing meals, System Organ Class, the highest level of the Grade 1 Mild; asymptomatic or mild shopping for groceries or clothes, using the MedDRA hierarchy, is identified by anatomical or symptoms; clinical or diagnostic telephone, managing money, etc. physiological system, etiology, or purpose (e.g., observations only; intervention not SOC Investigations for laboratory test results). indicated. **Self care ADL refer to bathing, dressing and CTCAE terms are grouped by MedDRA Primary Grade 2 Moderate; minimal, local or undressing, feeding self, using the toilet, taking SOCs.