Forestry Commission Bulletin: Management Of
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
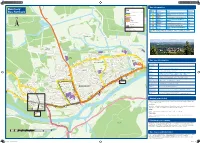
Banchory Bus Network
Bus Information A Banchory 9 80 Key Service Bus Network Bus services operating around Banchory Number Operator Route Operation 105 201 Stagecoach Aberdeen-Banchory-Aboyne-Ballater- Bluebird Braemar M-F, S, Su 201.202.203 202 Stagecoach 204 Bluebird Aberdeen-Banchory-Lumphanan/Aboyne M-F, S, Su Brathens VH5PM VH3 203 Stagecoach Aberdeen-Banchory/Aboyne/Ballater/ Wood Bluebird Braemar M-F VH5PM 204 Stagecoach Direction of travel Bluebird Aberdeen-Banchory-Strachan M-F ©P1ndar Bus stop VH3 Deeside Tarland-Aboyne-Finzean-Banchory Thu Building Drumshalloch Contains Ordnance Survey data VH5 Aboyne-Lumphanan-Tarland/Banchory © Crown copyright 2016 Deeside Circular F A980 Wood Digital Cartography by Pindar Creative www.pindarcreative.co.uk 01296 390100 Key: M-F - Monday to Friday Thu - Thursday F - Friday S - Saturday Su - Sunday Locton of Leys Upper Locton Wood VH5PM Upper Banchory Woodend Barn Locton Business Arts Centre Centre Biomass Road ’Bennie Energy Burn O Centre Business h ©P1ndar rc Tree C Centre a re L s ce t ©P1ndar n Pine Tree ry Eas H t ho Business il A Road ill of Banc l o 9 ©P1ndar H Centre f 8 B 0 ©P1ndar 201.202.203 ancho Raemoir 203 Pine Tree 201.202.203 Larch Tree Road ry Garden Centre d ©P1ndar E Crescent a a 203 o Hill of ©P1ndar s Oak Tree ©P1ndar R t y West e Banchory Avenue Hill of Banchor Larch Tree e ©P1ndar r Burn of Raemoir ©P1ndar Crescent Pine T Hill of Bus fare information Garden Sycamore ©P1ndar Bennie ©P1ndar Banchory ©P1ndar Centre Place ©P1ndar Sycamore Oak Tree Hill of Banchory Place Tesco Avenue ©P1ndar 203 est Tesco W d ry a Holly Tree ho 201.202 o VH5PM anc e R Ticket type Road f B Tre VH5PM ©P1ndar o aird’s W ll ne 201.202.203 C y i h Pi nd H t u ent VH5PM o resc Tesco S C ©P1ndar ©P1ndar stnut y he Single For a one-way journey, available on the bus. -

The Biology and Management of the River Dee
THEBIOLOGY AND MANAGEMENT OFTHE RIVERDEE INSTITUTEofTERRESTRIAL ECOLOGY NATURALENVIRONMENT RESEARCH COUNCIL á Natural Environment Research Council INSTITUTE OF TERRESTRIAL ECOLOGY The biology and management of the River Dee Edited by DAVID JENKINS Banchory Research Station Hill of Brathens, Glassel BANCHORY Kincardineshire 2 Printed in Great Britain by The Lavenham Press Ltd, Lavenham, Suffolk NERC Copyright 1985 Published in 1985 by Institute of Terrestrial Ecology Administrative Headquarters Monks Wood Experimental Station Abbots Ripton HUNTINGDON PE17 2LS BRITISH LIBRARY CATALOGUING-IN-PUBLICATIONDATA The biology and management of the River Dee.—(ITE symposium, ISSN 0263-8614; no. 14) 1. Stream ecology—Scotland—Dee River 2. Dee, River (Grampian) I. Jenkins, D. (David), 1926– II. Institute of Terrestrial Ecology Ill. Series 574.526323'094124 OH141 ISBN 0 904282 88 0 COVER ILLUSTRATION River Dee west from Invercauld, with the high corries and plateau of 1196 m (3924 ft) Beinn a'Bhuird in the background marking the watershed boundary (Photograph N Picozzi) The centre pages illustrate part of Grampian Region showing the water shed of the River Dee. Acknowledgements All the papers were typed by Mrs L M Burnett and Mrs E J P Allen, ITE Banchory. Considerable help during the symposium was received from Dr N G Bayfield, Mr J W H Conroy and Mr A D Littlejohn. Mrs L M Burnett and Mrs J Jenkins helped with the organization of the symposium. Mrs J King checked all the references and Mrs P A Ward helped with the final editing and proof reading. The photographs were selected by Mr N Picozzi. The symposium was planned by a steering committee composed of Dr D Jenkins (ITE), Dr P S Maitland (ITE), Mr W M Shearer (DAES) and Mr J A Forster (NCC). -

Land Management Plan
Deeside Woods LMP Moray & Aberdeenshire Forest District Banchory Woods Land Management Plan Plan Reference No: LMP 35 Plan Approval Date: Plan Expiry Date: 1 | Banchory Woods LMP 2018 -2027 | M Reeve | November 2018 Banchory Woods Land Management Plan 2018 - 2027 2 | Banchory Woods LMP 2018 – 2027 | M Reeve | November 2018 Banchory Woods Land Management Plan 2018 - 2027 FOREST ENTERPRISE - Application for Forest Design Plan Approvals in Scotland Forest Enterprise - Property Forest District: Moray & Aberdeenshire FD Woodland or property name: Banchory Woods Nearest town, village or locality: Banchory OS Grid reference: NO 688 948 Areas for approval Conifer Broadleaf Clear felling 196.9ha 3.2ha Selective felling 11.5ha Restocking 117.0ha 133.0ha New planting (complete appendix 4) 1. I apply for Forest Design Plan approval*/amendment approval* for the property described above and in the enclosed Forest Design Plan. 2. * I apply for an opinion under the terms of the Environmental Impact Assessment (Forestry) (Scotland) Regulations 1999 for afforestation* /deforestation*/ roads*/ quarries* as detailed in my application. 3. I confirm that the initial scoping of the plan was carried out with FC staff on July 2015 4. I confirm that the proposals contained in this plan comply with the UK Forestry Standard. 5. I confirm that the scoping, carried out and documented in the Consultation Record attached, incorporated those stakeholders which the FC agreed must be included. 6. I confirm that consultation and scoping has been carried out with all relevant stakeholders over the content of the of the design plan. Consideration of all of the issues raised by stakeholders has been included in the process of plan preparation and the outcome recorded on the attached consultation record. -

International Passenger Survey, 2008
UK Data Archive Study Number 5993 - International Passenger Survey, 2008 Airline code Airline name Code 2L 2L Helvetic Airways 26099 2M 2M Moldavian Airlines (Dump 31999 2R 2R Star Airlines (Dump) 07099 2T 2T Canada 3000 Airln (Dump) 80099 3D 3D Denim Air (Dump) 11099 3M 3M Gulf Stream Interntnal (Dump) 81099 3W 3W Euro Manx 01699 4L 4L Air Astana 31599 4P 4P Polonia 30699 4R 4R Hamburg International 08099 4U 4U German Wings 08011 5A 5A Air Atlanta 01099 5D 5D Vbird 11099 5E 5E Base Airlines (Dump) 11099 5G 5G Skyservice Airlines 80099 5P 5P SkyEurope Airlines Hungary 30599 5Q 5Q EuroCeltic Airways 01099 5R 5R Karthago Airlines 35499 5W 5W Astraeus 01062 6B 6B Britannia Airways 20099 6H 6H Israir (Airlines and Tourism ltd) 57099 6N 6N Trans Travel Airlines (Dump) 11099 6Q 6Q Slovak Airlines 30499 6U 6U Air Ukraine 32201 7B 7B Kras Air (Dump) 30999 7G 7G MK Airlines (Dump) 01099 7L 7L Sun d'Or International 57099 7W 7W Air Sask 80099 7Y 7Y EAE European Air Express 08099 8A 8A Atlas Blue 35299 8F 8F Fischer Air 30399 8L 8L Newair (Dump) 12099 8Q 8Q Onur Air (Dump) 16099 8U 8U Afriqiyah Airways 35199 9C 9C Gill Aviation (Dump) 01099 9G 9G Galaxy Airways (Dump) 22099 9L 9L Colgan Air (Dump) 81099 9P 9P Pelangi Air (Dump) 60599 9R 9R Phuket Airlines 66499 9S 9S Blue Panorama Airlines 10099 9U 9U Air Moldova (Dump) 31999 9W 9W Jet Airways (Dump) 61099 9Y 9Y Air Kazakstan (Dump) 31599 A3 A3 Aegean Airlines 22099 A7 A7 Air Plus Comet 25099 AA AA American Airlines 81028 AAA1 AAA Ansett Air Australia (Dump) 50099 AAA2 AAA Ansett New Zealand (Dump) -

A Guide to Biological Recording in Scotland
A Guide to Biological Recording t lan ' Produced by the Bipi gical Recording in ScotlaJ d Committee jj n i$ry.1977: Compiled By Alastgir Sommeryille~; PREFACE This manual is intended as a source of information about biological recording of all kinds, covering both the national distribution-mapping schemes and all of the other surveys and recording projects currently active in Scotland. It describes the aims of each scheme and gives an indication of the amount of work and degree of skill required from anybody wishing to participate. The appendices of addresses, reference works and distribution maps are intended to provide a useful way-in to potential recorders. It is hoped that bringing together all the schemes in this way will give readers of this manual some idea of the scope of the investigations being carried out and the part that they might play in improving the knowledge we have of the plants, animals and habitats of Scotland. BRISC would like to thank all the scheme organisers who have been so ready to enlighten us about their projects, the Biological Records Centre, Monks Wood for their support and the Nature Conservancy Council for the financial help to publish this manual. Any factual or implied errors in the text are, of course, entirely ours. CONTENTS Page National Distribution Mapping 5 chemes in progress - Summer 1976 6 Plants: Marine Algae 6 Larger Fungi 6 Lichen 9 Mosses and Liverworts 9 Ferns and Horsetails 9 Flowering Plants, Grasses and Ferns 9 Floras 10 Rare Plants 10 Plant Conservation 10 Animals: Marine Dinoflagellates -
I General Area of South Quee
Organisation Address Line 1 Address Line 2 Address Line3 City / town County DUNDAS PARKS GOLFGENERAL CLUB- AREA IN CLUBHOUSE OF AT MAIN RECEPTION SOUTH QUEENSFERRYWest Lothian ON PAVILLION WALL,KING 100M EDWARD FROM PARK 3G PITCH LOCKERBIE Dumfriesshire ROBERTSON CONSTRUCTION-NINEWELLS DRIVE NINEWELLS HOSPITAL*** DUNDEE Angus CCL HOUSE- ON WALLBURNSIDE BETWEEN PLACE AG PETERS & MACKAY BROS GARAGE TROON Ayrshire ON BUS SHELTERBATTERY BESIDE THE ROAD ALBERT HOTEL NORTH QUEENSFERRYFife INVERKEITHIN ADJACENT TO #5959 PEEL PEEL ROAD ROAD . NORTH OF ENT TO TRAIN STATION THORNTONHALL GLASGOW AT MAIN RECEPTION1-3 STATION ROAD STRATHAVEN Lanarkshire INSIDE RED TELEPHONEPERTH ROADBOX GILMERTON CRIEFFPerthshire LADYBANK YOUTHBEECHES CLUB- ON OUTSIDE WALL LADYBANK CUPARFife ATR EQUIPMENTUNNAMED SOLUTIONS ROAD (TAMALA)- IN WORKSHOP OFFICE WHITECAIRNS ABERDEENAberdeenshire OUTSIDE DREGHORNDREGHORN LOAN HALL LOAN Edinburgh METAFLAKE LTD UNITSTATION 2- ON ROAD WALL AT ENTRANCE GATE ANSTRUTHER Fife Premier Store 2, New Road Kennoway Leven Fife REDGATES HOLIDAYKIRKOSWALD PARK- TO LHSROAD OF RECEPTION DOOR MAIDENS GIRVANAyrshire COUNCIL OFFICES-4 NEWTOWN ON EXT WALL STREET BETWEEN TWO ENTRANCE DOORS DUNS Berwickshire AT MAIN RECEPTIONQUEENS OF AYRSHIRE DRIVE ATHLETICS ARENA KILMARNOCK Ayrshire FIFE CONSTABULARY68 PIPELAND ST ANDREWS ROAD POLICE STATION- AT RECEPTION St Andrews Fife W J & W LANG LTD-1 SEEDHILL IN 1ST AID ROOM Paisley Renfrewshire MONTRAVE HALL-58 TO LEVEN RHS OFROAD BUILDING LUNDIN LINKS LEVENFife MIGDALE SMOLTDORNOCH LTD- ON WALL ROAD AT -

Of 89 Filter
Filter: Page 2 of 89 Aberdeenshire Council List of Highways.a Transportation South Division South Highways List Stonehaven 25/03/2015 Roads Road No. Road Name Description Length Total Length of Roads (Miles) Filter: Page 3 of 89 Aberdeenshire Council List of Highways.a Transportation South Division South Highways List Stonehaven 25/03/2015 Trunk Roads Road No. Road Name Description Length A90(T) Perth - Aberdeen Road From Regional Boundary at North Water Bridge 30.276 round Laurencekirk By-pass, by Fordoun, Bridge of Mondynes and Fiddes, round Stonehaven By-pass, by Muchalls, Newtonhill, Cammachmore and Portlethen to District Boundary at Bothiebridge Total Length of Trunk Roads (Miles) 30.276 Filter: Page 4 of 89 Aberdeenshire Council List of Highways.a Transportation South Division South Highways List Stonehaven 25/03/2015 Class I Roads Road No. Road Name Description Length A92 Stonehaven - Montrose From Regional Boundary at Lower North Water 16.896 Road Bridge by St. Cyrus, Johnshaven, Inverbervie, (Montrose Road, King Street, Inverbervie) joining A90(T) North of Glasslaw Bridge, including slip roads on to A90(T). A93 North Deeside Road From District Boundary at Peterculter via Banchory 58.932 (Station Road, High Street, Inchmarlo Road, Banchory) thence via Bridge of Canny, Trustach, Aboyne (Ballater Road, Aboyne) thence to Ballater (Tullich Road, Bridge Street, Braemar Road, Ballater) thence to Braemar (Old Military Road, Glenshee Road, Braemar) thence to Regional Boundary at Cairnwell. A937 Mill of Conveth - From Regional Boundary at Marykirk Bridge, through 5.506 Laurencekirk - Montrose Marykirk, across A90(T) South of Laurencekirk, Road along High Street, Laurencekirk to junction with A90(T) North of Laurencekirk at Conveth Mains. -
Page 2 of 89 Filter
Filter: Page 2 of 89 Aberdeenshire Council List of Highways.a Transportation South Division South Highways List Stonehaven 11/07/2016 Trunk Roads Road No. Road Name Description Length A90(T) Perth - Aberdeen Road From Regional Boundary at North Water Bridge 30.276 round Laurencekirk By-pass, by Fordoun, Bridge of Mondynes and Fiddes, round Stonehaven By-pass, by Muchalls, Newtonhill, Cammachmore and Portlethen to District Boundary at Bothiebridge Total Length of Trunk Roads (Miles) 30.276 Filter: Page 3 of 89 Aberdeenshire Council List of Highways.a Transportation South Division South Highways List Stonehaven 11/07/2016 Class I Roads Road No. Road Name Description Length A92 Stonehaven - Montrose From Regional Boundary at Lower North Water 16.896 Road Bridge by St. Cyrus, Johnshaven, Inverbervie, (Montrose Road, King Street, Inverbervie) joining A90(T) North of Glasslaw Bridge, including slip roads on to A90(T). A93 North Deeside Road From District Boundary at Peterculter via Banchory 58.932 (Station Road, High Street, Inchmarlo Road, Banchory) thence via Bridge of Canny, Trustach, Aboyne (Ballater Road, Aboyne) thence to Ballater (Tullich Road, Bridge Street, Braemar Road, Ballater) thence to Braemar (Old Military Road, Glenshee Road, Braemar) thence to Regional Boundary at Cairnwell. A937 Mill of Conveth - From Regional Boundary at Marykirk Bridge, through 5.506 Laurencekirk - Montrose Marykirk, across A90(T) South of Laurencekirk, Road along High Street, Laurencekirk to junction with A90(T) North of Laurencekirk at Conveth Mains. A939 Deeside - Tomintoul Road From Route A93 at Bridge of Gairn via Gairnshiel to 8.743 District Boundary at Cairndoor. A957 Slug Road From A92 near Glasslaw, through Stonehaven, 14.848 (Dunnottar Avenue, Bridgefield, Allardice Street, Market Square South, Evan Street, Slug Road, Stonehaven) thence by Rickarton, Blackburn Bridge, Blairydryne and Durris Bridge to A93 at Baldarroch. -

Angus and Mearns Directory and Almanac, 1846
21 DAYS ALLOWED FOR READING THIS BOOK. Overdue Books Charged at Ip per Day. FORFAR PUBLIC LIBRARY IL©CAIL C©iLILECirD©IN ANGUS - CULTURAL SERVICES lllllllllillllllllllllllllllillllllllllllllllllllllllllllll Presented ^m . - 01:91^ CUStPI .^HE isms AND MSARNS ' DIRECTORY FOR 18^6 couni Digitized by tlie Internet Arcliive in 2010 witli funding from National Library of Scotland http://www.archive.org/details/angusmearnsdirec1846unse - - 'ir- AC'-.< u —1 >- GQ h- D >- Q. a^ LU 1*- <f G. O (^ O < CD i 1 Q. o U. ALEX MAC HABDY THE ANGUS AND MEAENS DIRECTORY FOR 1846, CONTAINING IN ADDITION TO THE WHOLE OP THE LISTS CONNECTED WITH THE COUNTIES OP FORFAR AND KINCARDINE, AND THE BURGHS OP DUNDEE, MONTROSE, ARBROATH, FORFAR, KIRRIEMUIR, STONEHAVEN, &c, ALPHABETICAL LISTS 'of the inhabitants op MONTROSE, ARBROATH, FORFAR, BRECBIN, AND KIRRIEMUIR; TOGETHEK WITH A LIST OF VESSELS REGISTERED AT THE PORTS OF MONTROSE, ARBROATH, DUNDEE, PERTH, ABERDEEN AND STONEHAVEN. MONTROSE PREPARED AND PUBLISHED BY JAMUI^ \VATT, STANDARD OFFICE, AND SOIiD BY ALL THE BOOKSELLERS IN THE TWO COUNTIES. EDINBURGH: BLACKWOOD & SON, AND OLIVER &c BOYD, PRINTED AT THE MONTROSE STANDARD 0FFIC5 CONTENTS. Page. Page Arbroath Dfrectory— Dissenting Bodies 178 Alphabetical List of Names 84 Dundee DtRECTORY— Banks, Public Offices, &c. 99 Banks, Public Offices, &c. 117 Burgh Funds . 102 Burgh Funds .... 122 Biiri^h Court 104 Banking Companies (Local) 126 128 Bible Society . • 105 Burgh Court .... Coaches, Carriers, &c. 100 Building Company, Joint-Stock 131 Comraerciiil Associations . 106 Coaches 11« Cliarities . , 106 Carriers 119 Educational Institutions . 104 Consols for Foreign States 121 Fire and Life Insurance Agents 101 Cemetery Company 124 Friendly Societies . -

New Series, Volume 19, 2018
NEW SERIES, VOLUME 19, 2018 DISCOVERY AND EXCAVATION IN SCOTLAND A’ LORG AGUS A’ CLADHACH AN ALBAINN NEW SERIES, VOLUME 19 2018 Editor Paula Milburn Archaeology Scotland Archaeology Scotland is a voluntary membership organisation, which works to secure the archaeological heritage of Scotland for its people through education, promotion and support: • education, both formal and informal, concerning Scotland’s archaeological heritage • promotion of the conservation, management, understanding and enjoyment of, and access to, Scotland’s archaeological heritage • support through the provision of advice, guidance, resources and information related to archaeology in Scotland Our vision Archaeology Scotland is the leading independent charity working to inspire people to discover, explore, care for and enjoy Scotland’s archaeological heritage. Our mission …to inspire the discovery, exploration, stewardship and enjoyment of Scotland’s past. Membership of Archaeology Scotland Membership is open to all individuals, local societies and organisations with an interest in Scottish archaeology. Membership benefits and services include access to a network of archaeological information on Scotland and the UK, three newsletters a year, the annual edition of the journal Discovery and excavation in Scotland, and the opportunity to attend Archaeology Scotland’s annual Summer School and the Archaeological Research in Progress conference. Further information and an application form may be obtained from Archaeology Scotland Email [email protected] Website www.archaeologyscotland.org.uk A’ lorg agus a’ cladhach an Albainn The Gaelic translation of Discovery and excavation in Scotland was supplied by Margaret MacIver, Lecturer in Gaelic and Education, and Professor Colm O’Boyle, Emeritus Professor, both at the Celtic, School of Language and Literature, University of Aberdeen. -

North East Local Plan District Local Flood Risk Management Plan
North East Local Plan District Local Flood Risk Management Plan Published by: Aberdeenshire Council, June 2016 Flood Risk Management (Scotland) Act 2009 North East Local Plan District Local Flood Risk Management Plan Delivering sustainable flood risk management is important for Scotland’s continued economic success and well-being. It is essential that we avoid and reduce the risk of flooding, and prepare and protect ourselves and our communities. This is the first Local Flood Risk Management Plan for the North East Local Plan District, describing the actions which will contribute to managing the risk of flooding and recovering from any future flood events. The task now for us – local authorities, Scottish Water, the Scottish Environment Protection Agency (SEPA), the Scottish Government and all other responsible authorities and public bodies is to deliver this plan. 2 Flood Risk Management (Scotland) Act 2009 North East Local Plan District Local Flood Risk Management Plan Foreword The impacts of flooding experienced by individuals, communities and businesses can be devastating and long lasting. It is vital that we continue to reduce the risk of any such future events and improve the area’s ability to manage and recover from any events which do occur. The publication of this Plan is an important milestone in implementing the Flood Risk Management (Scotland) Act 2009 and improving how we cope with and manage floods in the North East Local Plan District. The Plan translates this legislation into actions over the first planning cycle from 2016 to 2022 to reduce the risk of damage and distress caused by flooding. -

The Story of Muir of Dinnet National Nature Reserve
The Story of Muir of Dinnet National Nature Reserve 2nd Edition For further information on Muir of Dinnet NNR please contact: The Reserve Manager Scottish Natural Heritage Burn O’Vat Visitor Centre Dinnet Aboyne Aberdeenshire AB34 5NB Tel: 013398 81667 Email: [email protected] The Story of Muir of Dinnet National Nature Reserve The Story of Muir of Dinnet National Nature Reserve Foreword Muir of Dinnet National Nature Reserve (NNR) is a landscape shaped by retreating glaciers at the end of the last Ice Age around 15,000 years ago. The most striking feature is the ‘Vat’ - a giant pothole some 25 metres across formed by the glacial meltwaters. Throughout the reserve, the melting glaciers left behind a variety of deposits and meltwater channels and kettle hole depressions that form the basis for the variety of habitats and species found at Muir of Dinnet today. At the heart of the reserve are Lochs Davan and Kinord, with their near pure water and associated bogs and fens providing ideal habitat for a wide mix of species; from rare water beetles to the elusive otter, feeding and breeding on the reserve. During winter, the lochs are an important roost site, attracting migrating geese and other wildfowl. Dry heaths, including the internationally important bearberry heath, thrive on the drier hummocky ground. The heaths support rare moths, such as the netted mountain and cousin German, and in summer are home to ground nesting birds, such as curlew and meadow pipit. Young pine and birch now cover large areas of the reserve and provide an interesting transition from open heath, young scrub to pine and birch woodland.