Depicting the Mating System and Patterns of Contemporary Pollen flow in Trees of the Genus Anadenanthera (Fabaceae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
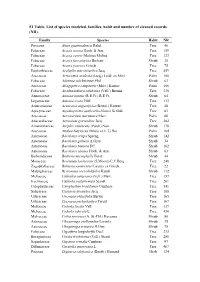
S1 Table. List of Species Modeled, Families, Habit and Number of Cleaned Records (NR)
S1 Table. List of species modeled, families, habit and number of cleaned records (NR). Family Species Habit NR Pinaceae Abies guatemalensis Rehd. Tree 46 Fabaceae Acacia aroma Hook. & Arn. Tree 159 Fabaceae Acacia caven (Molina) Molina Tree 123 Fabaceae Acacia furcatispina Burkart Shrub 35 Fabaceae Acacia praecox Griseb. Tree 75 Euphorbiaceae Acalypha macrostachya Jacq. Tree 459 Arecaceae Acrocomia aculeata (Jacq.) Lodd. ex Mart. Palm 166 Fabaceae Adesmia volckmannii Phil. Shrub 63 Arecaceae Allagoptera campestris (Mart.) Kuntze Palm 106 Fabaceae Anadenanthera colubrina (Vell.) Brenan Tree 330 Annonaceae Annona nutans (R.E.Fr.) R.E.Fr. Shrub 64 Loganiaceae Antonia ovata Pohl Tree 113 Araucariaceae Araucaria angustifolia (Bertol.) Kuntze Tree 48 Apocynaceae Aspidosperma quebracho-blanco Schltdl. Tree 83 Arecaceae Astrocaryum murumuru Mart. Palm 66 Anacardiaceae Astronium graveolens Jacq. Tree 282 Amaranthaceae Atriplex canescens (Pursh) Nutt. Shrub 178 Arecaceae Attalea butyracea (Mutis ex L.f.) We Palm 104 Asteraceae Baccharis crispa Spreng. Shrub 142 Asteraceae Baccharis gilliesii A.Gray Shrub 34 Asteraceae Baccharis trimera DC. Shrub 162 Asteraceae Baccharis ulicina Hook. & Arn. Shrub 63 Berberidaceae Berberis microphylla Forst. Shrub 44 Moraceae Brosimum lactescens (S.Moore) C.C.Berg Tree 248 Zygophyllaceae Bulnesia sarmientoi Lorentz ex Griseb. Tree 22 Malpighiaceae Byrsonima coccolobifolia Kunth Shrub 112 Meliaceae Cabralea canjerana (Vell.) Mart. Tree 157 Icacinaceae Calatola costaricensis Standl. Tree 201 Calophyllaceae Calophyllum brasiliense Cambess. Tree 541 Salicaceae Casearia decandra Jacq. Tree 188 Urticaceae Cecropia obtusifolia Bertol. Tree 165 Urticaceae Cecropia pachystachya Trécul Tree 167 Meliaceae Cedrela fissilis Vell. Tree 137 Meliaceae Cedrela odorata L. Tree 436 Malvaceae Ceiba speciosa (A. St.-Hil.) Ravenna Shrub 80 Asteraceae Chuquiraga avellanedae Lorentz Shrub 35 Asteraceae Chuquiraga erinacea D.Don Shrub 75 Fabaceae Copaifera langsdorffii Desf. -

Supplementary Materialsupplementary Material
10.1071/BT13149_AC © CSIRO 2013 Australian Journal of Botany 2013, 61(6), 436–445 SUPPLEMENTARY MATERIAL Comparative dating of Acacia: combining fossils and multiple phylogenies to infer ages of clades with poor fossil records Joseph T. MillerA,E, Daniel J. MurphyB, Simon Y. W. HoC, David J. CantrillB and David SeiglerD ACentre for Australian National Biodiversity Research, CSIRO Plant Industry, GPO Box 1600 Canberra, ACT 2601, Australia. BRoyal Botanic Gardens Melbourne, Birdwood Avenue, South Yarra, Vic. 3141, Australia. CSchool of Biological Sciences, Edgeworth David Building, University of Sydney, Sydney, NSW 2006, Australia. DDepartment of Plant Biology, University of Illinois, Urbana, IL 61801, USA. ECorresponding author. Email: [email protected] Table S1 Materials used in the study Taxon Dataset Genbank Acacia abbreviata Maslin 2 3 JF420287 JF420065 JF420395 KC421289 KC796176 JF420499 Acacia adoxa Pedley 2 3 JF420044 AF523076 AF195716 AF195684; AF195703 Acacia ampliceps Maslin 1 KC421930 EU439994 EU811845 Acacia anceps DC. 2 3 JF420244 JF420350 JF419919 JF420130 JF420456 Acacia aneura F.Muell. ex Benth 2 3 JF420259 JF420036 JF420366 JF419935 JF420146 KF048140 Acacia aneura F.Muell. ex Benth. 1 2 3 JF420293 JF420402 KC421323 JQ248740 JF420505 Acacia baeuerlenii Maiden & R.T.Baker 2 3 JF420229 JQ248866 JF420336 JF419909 JF420115 JF420448 Acacia beckleri Tindale 2 3 JF420260 JF420037 JF420367 JF419936 JF420147 JF420473 Acacia cochlearis (Labill.) H.L.Wendl. 2 3 KC283897 KC200719 JQ943314 AF523156 KC284140 KC957934 Acacia cognata Domin 2 3 JF420246 JF420022 JF420352 JF419921 JF420132 JF420458 Acacia cultriformis A.Cunn. ex G.Don 2 3 JF420278 JF420056 JF420387 KC421263 KC796172 JF420494 Acacia cupularis Domin 2 3 JF420247 JF420023 JF420353 JF419922 JF420133 JF420459 Acacia dealbata Link 2 3 JF420269 JF420378 KC421251 KC955787 JF420485 Acacia dealbata Link 2 3 KC283375 KC200761 JQ942686 KC421315 KC284195 Acacia deanei (R.T.Baker) M.B.Welch, Coombs 2 3 JF420294 JF420403 KC421329 KC955795 & McGlynn JF420506 Acacia dempsteri F.Muell. -

The Genus Anadenanthera in Amerindian Cultures
THE GENUS ANADENANTHERA IN AMERINDIAN CULTURES BY SIRI VON REIS ALTSCHUL, PH .D. RESEARCH FELLOW BOTANICAL MUSEUM HARVARD UNIVERSITY CAMBRIDGE, MASSACHUSETTS 1972 This monograph is dedicated in affectionate memory to the late DANIEL HERBERT EFRON, PH.D., M.D. 1913-1972 cherished friend of the author and of the Botanical Museum, a true scientist devoted to the interdisciplinary approach in the advancement of knowledge. A/""'f""'<J liz {i>U<// t~ },~u 0<<J4 ~ If;. r:J~ ~ //"'~uI ~ ()< d~ ~ !dtd't;:..1 "./.u.L .A Vdl0 If;;: ~ '" OU'''-k4 :/" tu-d ''''''"-''t2.. ?,,".jd,~ jft I;ft'- ?_rl; A~~ ~r'4tft,t -5 " q,.,<,4 ~~ l' #- /""/) -/~ "1'Ii;. ,1""", "/'/1'1",, I X C"'-r'fttt. #) (../..d ~;, . W,( ~ ~ f;r"'" y it;.,,J 11/" Y 4J.. %~~ l{jr~ t> ~~ ~txh '1'ix r 4 6~" c/<'T'''(''-;{' rn« ?.d ~;;1';;/ a-.d txZ-~ ~ ;o/~ <A.H-iz "" ~".,/( 1-/X< "..< ,:" -.... ~ ~ . JJlr-0? on . .it-(,0.1' r 4 -11<.1.- aw./{') -:JL. P7t;;"j~;1 S .d-At ;0~/lAQ<..t ,ti~?,f,.... vj "7rU<-'- ~I""" =iiR-I1;M~ a....k«<-l, ¢- f!!) d..;.:~ M ~ ~y£/1 ~/.u..-... It'--, "" # :Z:-,k. "i ~ "d/~ efL<.<~/ ,w 1'#,') /';~~;d-t a;.. tlArl-<7'" I .Ii;'~.1 (1(-;.,} >Lc -(l"7C),.,..,;.. :.... ,,:/ ~ /-V,~ , ,1" # (i F'"' l' fJ~~A- (.tG- ~/~ Z:--7Co- ,,:. ,L7r= f,-, , ~t) ahd-p;: fJ~ / tr>d .4 ~f- $. b".,,1 ~/. ~ pd. 1'7'-· X ~-t;;;;.,~;z jM ~0Y:tJ;; ~ """.,4? br;K,' ./.n.u" ~ 7r .".,.~,j~ ;;f;tT ~ ..4'./ ;pf,., tJd~ M_~ (./I<'/~.'. IU. et. c./,. ~L.y !f-t.<H>:t;.tu ~ ~,:,-,p., .....:. -

Different Mechanisms Drive the Performance of Native and Invasive Woody Species in Response to Leaf Phosphorus Supply During Periods of Drought Stress and Recovery
Plant Physiology and Biochemistry 82 (2014) 66e75 Contents lists available at ScienceDirect Plant Physiology and Biochemistry journal homepage: www.elsevier.com/locate/plaphy Research article Different mechanisms drive the performance of native and invasive woody species in response to leaf phosphorus supply during periods of drought stress and recovery * Marciel Teixeira Oliveira, Camila Dias Medeiros, Gabriella Frosi, Mauro Guida Santos Departamento de Botanica,^ Laboratorio de Ecofisiologia Vegetal, Universidade Federal de Pernambuco, Recife PE 50670-901, Brasil article info abstract Article history: The effects of drought stress and leaf phosphorus (Pi) supply on photosynthetic metabolism in woody Received 31 March 2014 tropical species are not known, and given the recent global environmental change models that forecast Accepted 13 May 2014 lower precipitation rates and periods of prolonged drought in tropical areas, this type of study is Available online 22 May 2014 increasingly important. The effects of controlled drought stress and Pi supply on potted young plants of two woody species, Anadenanthera colubrina (native) and Prosopis juliflora (invasive), were determined Keywords: by analyzing leaf photosynthetic metabolism, biochemical properties and water potential. In the Chlorophyll fluorescence maximum stress, both species showed higher leaf water potential (Jl) in the treatment drought þPi Climate change À Drought tolerance when compared with the respective control Pi. The native species showed higher gas exchange under þ e Gas exchange drought Pi than under drought Pi conditions, while the invasive species showed the same values Water deficit between drought þPi and ÀPi. Drought affected the photochemical part of photosynthetic machinery more in the invasive species than in the native species. -

A Genetic Study on Subtropical Anadenanthera Colubrina (Vell.) Brenan Var
Journal of Forest Research ISSN: 1341-6979 (Print) 1610-7403 (Online) Journal homepage: http://www.tandfonline.com/loi/tjfr20 A genetic study on subtropical Anadenanthera colubrina (Vell.) Brenan var. cebil (Griseb.) Altschul tree from Northwestern Argentina Ciaccio Mirella, Russo Roberta, Palla Franco, Giamminola Eugenia & de Viana Marta Leonór To cite this article: Ciaccio Mirella, Russo Roberta, Palla Franco, Giamminola Eugenia & de Viana Marta Leonór (2017): A genetic study on subtropical Anadenanthera colubrina (Vell.) Brenan var. cebil (Griseb.) Altschul tree from Northwestern Argentina, Journal of Forest Research, DOI: 10.1080/13416979.2017.1283975 To link to this article: http://dx.doi.org/10.1080/13416979.2017.1283975 View supplementary material Published online: 07 Feb 2017. Submit your article to this journal Article views: 1 View related articles View Crossmark data Full Terms & Conditions of access and use can be found at http://www.tandfonline.com/action/journalInformation?journalCode=tjfr20 Download by: [Consiglio Nazionale delle Ricerche], [Roberta Russo] Date: 08 February 2017, At: 22:48 JOURNAL OF FOREST RESEARCH, 2017 http://dx.doi.org/10.1080/13416979.2017.1283975 A genetic study on subtropical Anadenanthera colubrina (Vell.) Brenan var. cebil (Griseb.) Altschul tree from Northwestern Argentina Ciaccio Mirella a, Russo Roberta a, Palla Francob, Giamminola Eugeniac and de Viana Marta Leonórc aIstituto di Biomedicina e Immunologia molecolare “A. Monroy”, Consiglio Nazionale delle Ricerche (CNR), Palermo, Italy; bDipartimento di Scienze e Tecnologie Biologiche, Chimiche e Farmaceutiche, Sezione Botanica ed Ecologia vegetale, Palermo, Italy; cBanco de Germoplasma de Especies Nativas, Instituto de Ecología y Ambiente Humano, Universidad Nacional de Salta, Salta, Argentina ABSTRACT ARTICLE HISTORY Anadenanthera colubrina (Vell.) Brenan var. -

Bifurcated Snuff Tubes in the Pre-Columbian Caribbean
Journal of Caribbean Archaeology Copyright 2020 ISBN 1524-4776 Conduits to the supernatural: Bifurcated snuff tubes in the pre-Columbian Caribbean Joanna Ostapkowicz School of Archaeology University of Oxford Oxford, OX1 2PG, UK [email protected] UK museum collections hold an important and largely unexplored corpus of Caribbean pre-Columbian cultural heritage, including seminal pieces that can offer new insights into the development of complex rituals in the region. This paper re-establishes the cultural context and significance of a previously undocumented carving related to cohoba drug rituals: an ornate, composite snuff tube carved of cannel coal, recovered from the Lesser Antillean island of St Vincent before 1870, and donated to Oxford's Pitt Rivers Museum in 1900. Both the material (which does not occur in the insular Caribbean) and the carving style suggest that the snuff tube was an import from Venezuela's Lower Orinoco region, where the Barrancoid style emerged in its classic form ca. ~100 BC - AD 500 (Los Barrancos complex). As such, it is the earliest example of drug paraphernalia often assumed to have been used only after ~AD 1000, and isolated to the chiefdom-level societies of the Greater Antilles. This paper contributes a brief review of the St Vincent snuff tube within the context of other stone and wood examples in public collections in efforts to explore their diagnostics, range and, ultimately, the ceremonies in which they were used. Las colecciones museísticas del Reino Unido conservan un importante y a su vez poco conocido corpus de materiales pertenecientes al patrimonio precolombino caribeño, entre los que se incluyen piezas destacadas que pueden ofrecer información sobre el desarrollo de rituales complejos en la región. -

A Guide to Lesser Known Tropical Timber Species July 2013 Annual Repo Rt 2012 1 Wwf/Gftn Guide to Lesser Known Tropical Timber Species
A GUIDE TO LESSER KNOWN TROPICAL TIMBER SPECIES JULY 2013 ANNUAL REPO RT 2012 1 WWF/GFTN GUIDE TO LESSER KNOWN TROPICAL TIMBER SPECIES BACKGROUND: BACKGROUND: The heavy exploitation of a few commercially valuable timber species such as Harvesting and sourcing a wider portfolio of species, including LKTS would help Mahogany (Swietenia spp.), Afrormosia (Pericopsis elata), Ramin (Gonostylus relieve pressure on the traditionally harvested and heavily exploited species. spp.), Meranti (Shorea spp.) and Rosewood (Dalbergia spp.), due in major part The use of LKTS, in combination with both FSC certification, and access to high to the insatiable demand from consumer markets, has meant that many species value export markets, could help make sustainable forest management a more are now threatened with extinction. This has led to many of the tropical forests viable alternative in many of WWF’s priority places. being plundered for these highly prized species. Even in forests where there are good levels of forest management, there is a risk of a shift in species composition Markets are hard to change, as buyers from consumer countries often aren’t in natural forest stands. This over-exploitation can also dissuade many forest willing to switch from purchasing the traditional species which they know do managers from obtaining Forest Stewardship Council (FSC) certification for the job for the products that they are used in, and for which there is already their concessions, as many of these high value species are rarely available in a healthy market. To enable the market for LKTS, there is an urgent need to sufficient quantity to cover all of the associated costs of certification. -

Inner Visions: Sacred Plants, Art and Spirituality
AM 9:31 2 12/10/14 2 224926_Covers_DEC10.indd INNER VISIONS: SACRED PLANTS, ART AND SPIRITUALITY Brauer Museum of Art • Valparaiso University Vision 12: Three Types of Sorcerers Gouache on paper, 12 x 16 inches. 1989 Pablo Amaringo 224926_Covers_DEC10.indd 3 12/10/14 9:31 AM 3 224926_Text_Dec12.indd 3 12/12/14 11:42 AM Inner Visions: Sacred Plants, Art and Spirituality • An Exhibition of Art Presented by the Brauer Museum • Curated by Luis Eduardo Luna 4 224926_Text.indd 4 12/9/14 10:00 PM Contents 6 From the Director Gregg Hertzlieb 9 Introduction Robert Sirko 13 Inner Visions: Sacred Plants, Art and Spirituality Luis Eduardo Luna 29 Encountering Other Worlds, Amazonian and Biblical Richard E. DeMaris 35 The Artist and the Shaman: Seen and Unseen Worlds Robert Sirko 73 Exhibition Listing 5 224926_Text.indd 5 12/9/14 10:00 PM From the Director In this Brauer Museum of Art exhibition and accompanying other than earthly existence. Additionally, while some objects publication, expertly curated by the noted scholar Luis Eduardo may be culture specific in their references and nature, they are Luna, we explore the complex and enigmatic topic of the also broadly influential on many levels to, say, contemporary ritual use of sacred plants to achieve visionary states of mind. American and European subcultures, as well as to contemporary Working as a team, Luna, Valparaiso University Associate artistic practices in general. Professor of Art Robert Sirko, Valparaiso University Professor We at the Brauer Museum of Art wish to thank the Richard E. DeMaris and the Brauer Museum staff present following individuals and agencies for making this exhibition our efforts of examining visual products arising from the possible: the Brauer Museum of Art’s Brauer Endowment, ingestion of these sacred plants and brews such as ayahuasca. -

Richard Spruce: an Early Ethnobotanist and Explorer of the Northwest Amazon and Northern Andes
J. Ethnobiol. 3(2):139-147 December 1983 RICHARD SPRUCE: AN EARLY ETHNOBOTANIST AND EXPLORER OF THE NORTHWEST AMAZON AND NORTHERN ANDES RICHARD EVANS SCHULTES Jeffrey Professor of Biology and Director, Botanical Museum of Harvard University, Cambridge, Massachusetts 02138 ABSTRACT.-Although Richard Spruce, a pioneer botanical explorer of the northwest Amazon and the northern Andes in the middle of the last century, made numerous impor tant ethnobotanical discoveries, he missed the opportunity of delving deeply into the ethno pharmacological lore of the area. Part of this deficiency was due not certainly to his scien tific curiosity but to his inability to associate closely with unacculturated natives, the incredible intensity of his floristic and taxonomic studies, and possibly to his illness. Yet his research set the stage for recent ethnobotanical investigations of this very species-rich region. INTRODUCTION The Indians of the northwest Amazon, especially those of the Brazilian and Colom bian region of the Rfo Vaupes, have a rich ethnopharmacological lore. This wealth of knowledge of the presumed medicinal properties of plants, however, is just coming to the fore-and most certainly not too soon with its disappearance in the face of advancing acculturation and the inroads of civilization. Richard Spruce (Fig. I), the British plant-explorer who opened up this region to science between 1851 and 1854, must be counted amongst the greatest naturalists ever to have engaged in collecting and studies anywhere in virgin tropical American territories. As a result of his meticulous observation and insatiable curiosity, a basis for our under standing of great areas of the Amazon Valley and of the northern Andes was early and most firmly laid. -

Chemical Evidence for the Use of Multiple Psychotropic Plants in a 1,000-Year-Old Ritual Bundle from South America
Chemical evidence for the use of multiple psychotropic plants in a 1,000-year-old ritual bundle from South America Melanie J. Millera,b,1, Juan Albarracin-Jordanc, Christine Moored, and José M. Caprilese,1 aDepartment of Anatomy, University of Otago, Dunedin 9016, New Zealand; bArchaeological Research Facility, University of California, Berkeley, CA 94720; cInstituto de Investigaciones Antropológicas y Arqueológicas, Universidad Mayor de San Andrés, La Paz, Bolivia; dImmunalysis Corporation, Pomona, CA 91767; and eDepartment of Anthropology, The Pennsylvania State University, University Park, PA 16802 Edited by Linda R. Manzanilla, Universidad Nacional Autonóma de México, Mexico, D.F., Mexico, and approved April 9, 2019 (received for review February 6, 2019) Over several millennia, various native plant species in South these resources together. Using liquid chromatography tandem America have been used for their healing and psychoactive prop- mass spectrometry (LC-MS/MS), we tested for the presence of erties. Chemical analysis of archaeological artifacts provides an psychoactive compounds in the materials that composed a 1,000- opportunity to study the use of psychoactive plants in the past year-old ritual bundle excavated in a dry rock shelter from and to better understand ancient botanical knowledge systems. southwestern Bolivia. Liquid chromatography tandem mass spectrometry (LC-MS/MS) was used to analyze organic residues from a ritual bundle, radio- The Ritual Bundle from the Cueva del Chileno carbon dated to approximately 1,000 C.E., recovered from archae- The Sora River valley, located in the Lípez highlands of south- ological excavations in a rock shelter located in the Lípez Altiplano western Bolivia, is a narrow basin outlined by two parallel ig- of southwestern Bolivia. -

Morphological and Physiological Germination Aspects of Anadenanthera Colubrina (Vell.) Brenan
Available online: www.notulaebotanicae.ro Print ISSN 0255-965X; Electronic 1842-4309 Notulae Botanicae Horti AcademicPres Not Bot Horti Agrobo, 2018, 46(2):593-600. DOI:10.15835/nbha46211094 Agrobotanici Cluj-Napoca Original Article Morphological and Physiological Germination Aspects of Anadenanthera colubrina (Vell.) Brenan Fernanda Carlota NERY 1*, Marcela Carlota NERY 2, Débora de Oliveira PRUDENTE 3, Amauri Alves de ALVARENGA 3, Renato PAIVA 3 1Universidade Federal de São João Del Rei (UFSJ), Departamento de Engenharia de Biossistemas, São João Del Rei - MG, Brazil; [email protected] (*corresponding author) 2Universidade dos Vales do Jequitinhonha e Mucuri (UFVJM), Diamantina – MG, Brazil; [email protected] 3Universidade Federal de Lavras (UFLA), Programa de Pós-graduação em Fisiologia Vegetal, Departamento de Biologia, Lavras – MG, Brazil; [email protected] ; [email protected] ; [email protected] Abstra ct Anadenanthera colubrina is a species native to Brazil, from the Fabaceae family and has potential for use in the timber industry, in the reforestation of degraded areas, besides having medicinal properties. Its propagation is mainly by seeds, but basic subsidies regarding the requirements for optimal germination conditions are still lacking. Aiming to contribute to the expansion of its cultivation, rational use and conservation, the objective of this study was to investigate the morphology and anatomy of fruits and seeds, as well as the responses to factors as thermal regimes and substrates in seed germination. The 1000-seed weight and seeds per fruit were determined. To characterize the seed tissues, histochemical test with Sudan III and Lugol was used. The temperatures analyzed in the germination test were 15-25 °C; 25 °C; 20-30 °C and 30 °C. -
![IVEW3ITY NO. CXCIII a TAXONO]L'lic STUDY of the GE!\CS](https://docslib.b-cdn.net/cover/1498/ivew3ity-no-cxciii-a-taxono-llic-study-of-the-ge-cs-1501498.webp)
IVEW3ITY NO. CXCIII a TAXONO]L'lic STUDY of the GE!\CS
COi\TI:II)UTIONS FI:O~l THEGI:AY II ERB .~ R lml Of IlAl:VAltD U;\IV EW3ITY J::. lilt".1 b~· lh't',l c. Hl'Jl!i n ~ ull.I Hobtc n c. !-"ht, ~ NO. CXCIII A TAXONO]l'lIC STUDY OF THE GE!\CS ANADE Ai:'i THERA By S ItU Y01'\ REI S ALTSCHl"L Il\FRASPECIFIC VARIATION IN THE GUTlERREZIA SAROTHRAE CmlPLEX (COMPOSITAE-ASTEEEAE) By Orro T. SOLBRIG Published by THE GR AY HERBARIUlII OF HARVARD UKIVERSITY CAMBRIDGE, MASS., U. S. A. , MANUFACTURED BY THE LEXINGTON PRESS, INC. LEXINGTON. MASSACHUSETTS A TAX O:\O,llC STUDY OF TilE GE~ljS AN A DENA:\TI-IEI~A SIR! VO:-i HE!S ALT~l-HUL The "nail, l ropical to suutropical, and strictly :\ew World genu s .AlIlLl!C"1Wllth c,'n formerly was I...'ons ide}' ~d n~ $~c tion NioJ)(I of the genus Pip to.(/ cllia and i$ the cor.',m on It.'gumi 11 0 U S sou rce of lhe so-called narcotics known as COIIO/>O, l-iica a nd Yopo, Because of lheir ullusual c"rects upon th~ human nervollS system, the chemical constitutt.'nts of t~.ese material s are of cUlTen t interest in the field of ~xper im " nta l psychia lry, Among the chemical compound, which leave u.een iso lated from the species of A,wde1U",th ,Ta, may be included some hallucinogenic cl!'ugs designated as psychotomimetics (Hofmann, 1959) , In contrast to otlwr psychotropic drugs, which merely evoke mod ifications in the mood of th~ reci pi ent, the psychotomimetics appeal' to produce profound and acute changes in perception_ An interest both in plants of possibl~ medical significance and in species in need of t"xonomic stud,' provided a stimulus for the research whose results are published in part in this paper.