Readily Dismissed on Several Grounds.8-10
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mackenzie's Mission Gene & Condition List
Mackenzie’s Mission Gene & Condition List What conditions are being screened for in Mackenzie’s Mission? Genetic carrier screening offered through this research study has been carefully developed. It is focused on providing people with information about their chance of having children with a severe genetic condition occurring in childhood. The screening is designed to provide genetic information that is relevant and useful, and to minimise uncertain and unclear information. How the conditions and genes are selected The Mackenzie’s Mission reproductive genetic carrier screen currently includes approximately 1300 genes which are associated with about 750 conditions. The reason there are fewer conditions than genes is that some genetic conditions can be caused by changes in more than one gene. The gene list is reviewed regularly. To select the conditions and genes to be screened, a committee comprised of experts in genetics and screening was established including: clinical geneticists, genetic scientists, a genetic pathologist, genetic counsellors, an ethicist and a parent of a child with a genetic condition. The following criteria were developed and are used to select the genes to be included: • Screening the gene is technically possible using currently available technology • The gene is known to cause a genetic condition • The condition affects people in childhood • The condition has a serious impact on a person’s quality of life and/or is life-limiting o For many of the conditions there is no treatment or the treatment is very burdensome for the child and their family. For some conditions very early diagnosis and treatment can make a difference for the child. -

Iron Deficiency Anemia (IRIDA) • Rare
Iron Deficiency: Review Melinda Wu, MD, MCR Oregon Health & Science University OHSU10/17/2019 Disclosure Information: a) Moderators/panelists/presenters: Melinda Wu has nothing to disclose. OHSUb) Funding sources: NIH/NHLBI- K08 HL133493 Objectives 1) To review iron body homeostasis 2) To review the etiologies of iron deficiency OHSU3) To review various treatment options of iron deficiency Part I: Review of Iron Body OHSUHomeostasis Iron Balance in the Body Iron is required for growth of all cells, not just hemoglobin! Heme proteins: cytochromes, catalase, peroxidase, cytochrome oxidase Flavoproteins: cytochrome C reductase, succinic dehydrogenase, NADH oxidase, xanthine oxidase Too little Too much Not enough for essential Accumulates in organs proteins: Promotes the formation of: • Hemoglobin • Oxygen radicals •OHSURibonucleotide reductase • Lipid peroxidation (DNA synthesis) • DNA damage • Cytochromes • Tissue fibrosis • Oxidases Iron Economy • The average adult has 4-5 g of body iron. • ~10% of dietary iron absorbed, exclusively in duodenum • Varies with: • Iron content of diet • Bioavailability of dietary iron • Iron stores in body • Erythropoietic demand • Hypoxia • Inflammation • More than half is incorporated into erythroid precursors/mature erythrocytes OHSU• Only ~1-2 mg of iron enters and leaves the body in a day on average. • About 1 mg of iron is lost daily in menstruating women. Lesjak, M.; K. S. Srai, S. Role of Dietary Flavonoids in Iron Homeostasis. Pharmaceuticals 2019 Systemic Iron Regulation: Absorption Iron status is regulated entirely at the level of absorption! • Heme iron (30-70%) > non-heme iron (<5%) • 2 stable oxidation states: Ferrous (Fe 2+) > Ferric (Fe 3+) • Elemental iron must be reduced to Fe2+ iron to be absorbed 1. -

Mechanisms of Copper Deficiency in the Zebrafish Embryo Erik Madsen Washington University in St
Washington University in St. Louis Washington University Open Scholarship All Theses and Dissertations (ETDs) January 2010 Mechanisms of Copper Deficiency in the Zebrafish Embryo Erik Madsen Washington University in St. Louis Follow this and additional works at: https://openscholarship.wustl.edu/etd Recommended Citation Madsen, Erik, "Mechanisms of Copper Deficiency in the Zebrafish Embryo" (2010). All Theses and Dissertations (ETDs). 220. https://openscholarship.wustl.edu/etd/220 This Dissertation is brought to you for free and open access by Washington University Open Scholarship. It has been accepted for inclusion in All Theses and Dissertations (ETDs) by an authorized administrator of Washington University Open Scholarship. For more information, please contact [email protected]. WASHINGTON UNIVERSITY Division of Biology and Biomedical Sciences Molecular Cell Biology Dissertation Examination Committee Jonathan Gitlin, Chair John Atkinson Guojun Bu Aaron DiAntonio Steven Johnson Jeanne Nerbonne David Wilson MECHANISMS OF COPPER DEFICIENCY IN THE ZEBRAFISH EMBRYO by Erik Christian Madsen A dissertation presented to the Graduate School of Arts and Sciences of Washington University in partial fulfillment of the requirements for the degree of Doctor of Philosophy May 2010 Saint Louis, Missouri ABSTRACT OF THE DISSERTATION Mechanisms of Copper Deficiency in the Zebrafish Embryo By Erik Christian Madsen Doctor of Philosophy in Biology and Biomedical Sciences (Molecular Cell Biology) Washington University in St. Louis, 2010 Professor Jonathan D. Gitlin, Chairperson Proper maternal nutrition is critical for early embryonic development. Despite overwhelming epidemiologic data indicating the benefits nutrient supplementation for the developing organism we do not fully understand the genetics of predisposition to abnormal developmental phenotypes when faced with suboptimal nutrient levels. -

25. C:\Documents and Settings\Kwang-Il\My
The human disease network Goh K-I, Cusick ME, Valle D, Childs B, Vidal M, Barabasi′ A-L (2007) Proc Natl Acad Sci USA 104:8685-8690 Disorder Class Bone Coats Cancer Urolithiasise Osteopetrosis disease NDP Caffey van_Buchem Exudative Cardiovascular disease disease vitreoretinopathy Norrie SLC34A1 disease 439 LRP5 Connective tissue disorder Nevo Hyperostosis, syndrome COL1A1 endosteal Dermatological PLOD1 217 PAX9 Oligodontia Osteogenesis Osteoporosis 1164 Developmental Ehlers-Danlos imperfecta syndrome Arthropathy COL3A1 Hypodontia Ear, Nose, Throat Aneurysm, COL1A2 familial_arterial Myasthenic Witkop 733 syndrome Heart syndrome Pseudoachondroplasia Endocrine 3-methylglutaconicaciduria OPA3 WISP3 Optic Marfan block MSX1 atrophy OPA1 Aortic syndrome Paramyotonia Sick_sinus Gastrointestinal aneurysm congenita syndrome 3558 Intervertebral_disc Brugada SCN4A disease syndrome Syndactyly Spondyloepiphyseal COMP COL9A2 Hematological Glaucoma Weill-Marchesani Shprintzen-Goldberg Cramps, SCN5A Zlotogora-Ogur Cleft dysplasia syndrome syndrome potassium-aggravated Myotonia 2785 syndrome palate Parkes_Weber Basal_cell FBN1 congenita Oculodentodigital COL9A3 1432 Immunological 1414 CYP1B1 syndrome nevus_syndrome MASS Hypokalemic Acquired dysplasia Peters long_QT_syndrome Epiphyseal FLNB RASA1 PTCH Keratitis syndrome periodic MATN3 Metabolic SHH anomaly Eye Ectopia Thyrotoxic paralysis dysplasia Atelosteogenesis anomalies Marshall Larson Capillary Basal_cell Holoprosencephaly Coloboma, periodic KCNH2 PVRL1 malformations GJA1 Incontinentia syndrome SLC26A2 -

For Hemochromatosis (Atransferrinemia/Hemosiderosis/Iron Absorption) C
Proc. Natl. Acad. Sci. USA Vol. 84, pp. 3457-3461, May 1987 Medical Sciences Tissue distribution and clearance kinetics of non-transferrin-bound iron in the hypotransferrinemic mouse: A rodent model for hemochromatosis (atransferrinemia/hemosiderosis/iron absorption) C. M. CRAVEN*, J. ALEXANDERt, M. ELDRIDGE*, J. P. KUSHNERt, S. BERNSTEINt, AND J. KAPLAN*§ Departments of *Pathology and tMedicine, University of Utah College of Medicine, Salt Lake City, UT 84132; and tThe Jackson Laboratories, Bar Harbor, ME 04609 Communicated by Gilbert Ashwell, January 29, 1987 (receivedfor review October 28, 1986) ABSTRACT Genetically hypotransferrinemic mice accumu- (S.B., unpublished data; see refs. 9-11). These mice (HP) late iron in the liver and pancreas. A similar pattern oftissue iron were found to have a hypochromic microcytic anemia and accumulation occurs in humans with hereditary hemohroma- were growth-retarded at birth. Neonatal HP homozygotes die tosis. In both disorders, there is a decreased plasma concentration soon after birth but can be life-spared by weekly injections of of apotransferrin. To test the hypothesis that nontransferrin- whole mouse serum or Tf, even though the serum Tf levels bound iron exists and is cleared by the parenchymal tissues, the in the life-spared animals rarely exceeded 1% of normal tissue distribution of "9Fe was studied in animals lacking values. The life-spared adults develop parenchymal iron apotransferrin. Two groups of animal were used: normal rats overload with massive iron deposition in the liver and and mice whose transferrin had been saturated by an intravenous pancreas. The parenchymal iron accumulation was not injection of nonradiolabeled iron, and mice with congenital caused simply by the serum injections, as increased tissue hypotransferrinemia. -

Download CGT Exome V2.0
CGT Exome version 2. -
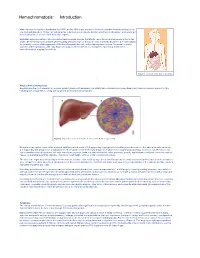
Hemochromatosis: Introduction
Hemochromatosis: Introduction Hemochromatosis was first identified in the 1800s, and by 1935 it was understood to be an inherited disease resulting in iron overload and deposition. Today, hemochromatosis is defined as a metabolic disorder affecting iron absorption , and resulting in the accumulation of excess iron in the body’s organs. Hereditary hemochromatosis (HH) is an autosomal recessive disorder that affects one in three hundred people in the United States. One in nine people carry the gene —making hemochromatosis the most common genetic disorder in the United States. It is primarily seen in middle-aged men of Northern European descent, and postmenopausal women. Treatment is readily available and the prognosis , with early diagnosis and proactive treatment, is a normal life expectancy. If untreated, hemochromatosis may lead to cirrhosis . Figure 1. Location of the liver in the body. What is Hemochromatosis? Despite being the most prevalent monoallelic genetic disease in Caucasians, hereditary hemochromatosis is under-diagnosed. There are several reasons for this, including lack of awareness, a long latency period, and nonspecific symptoms. Figure 2. Deposition of iron in the liver; A, Gross liver; B, Histological view. Normal iron absorption occurs in the proximal small intestine at a rate of 1-2 mg per day. In people with hereditary hemochromatosis, this absorption rate can reach 4–5 mg per day with progressive accumulation to 15–40 grams of iron in the body (Figure 2). Humans have no physiologic pathway to excrete iron. Therefore, iron can accumulate in any body tissue, although depositions are most common in the liver, thyroid, heart, pancreas, gonads, hypothalamus and joints. -

Genomeposter2009.Pdf
Fold HumanSelected Genome Genes, Traits, and Landmarks Disorders www.ornl.gov/hgmis/posters/chromosome genomics.energy. -

Congenital Hyperferritinemia Diagnosed in a 2 Month Old-A Case Report from India
MOUSHUMI LODH, JOSHI ANAND KERKETTA CONGENITAL HYPERFERRITINEMIA DIAGNOSED IN A 2 MONTH OLD-A CASE REPORT FROM INDIA The Journal of the International Federation of Clinical Chemistry and Laboratory Medicine CONGENITAL HYPERFERRITINEMIA DIAGNOSED IN A 2 MONTH OLD-A CASE REPORT FROM INDIA Moushumi Lodh1, Joshi Anand Kerketta2 1M.D Biochemistry, Senior Consultant, Department of Biochemistry, The Mission Hospital, Durgapur, West Bengal, India 2M.D Paediatrics, Consultant, Department of Paediatrics and Neonatology, the Mission Hospital, Durgapur, West Bengal, India Corresponding Author: Dr Moushumi Lodh, M.D Biochemistry Senior Consultant Department of Biochemistry The Mission Hospital, Immon Kalyan Sarani sector 2C, Bidhannagar Durgapur, West Bengal, India. Pin‐713212 Tel.: +91‐9800881640 Fax: 0343‐2532550 e‐mail address: [email protected] KEY WORDS Hyperferritinemia; iron overload; genetic hyperferritinemia; HHCS. LIST OF DECLARATIONS No authors declared any potential conflicts of interest. No funding and no ethical approval was required for this case report.The corresponding author is guarantor. All authors confirmed they have contributed to the intellectual content of this paper and have met the following requirements :(a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; and (c) final approval of the published article. RUNNING TITLE Congenital hyperferritinemia in 2 month old baby. ABSTRACT Background: In clinical medicine, ferritin is predominantly utilized as a serum marker of total body iron stores. In cases of iron deficiency and overload, serum ferritin serves a critical role in both diagnosis and management. Elevated serum and tissue ferritin are linked to coronary artery disease, malignancy, and poor outcomes following stem cell transplantation. -

Classification and Diagnosis of Iron Overload
Haematologica 1998; 83:447-455 decision making and problem solving Classification and diagnosis of iron overload ALBERTO PIPERNO Istituto di Scienze Biomediche, Azienda Ospedaliera S. Gerardo, Divisione di Medicina 1, Monza, Italy Abstract Background and Objective. Iron overload is the result ron overload is the result of many disorders and of many disorders and could lead to the development could lead to the development of organ damage of organ damage and increased mortality. The recent Iand increased mortality. The recent description description of new conditions associated with iron of new conditions associated with iron overload and overload and the identification of the genetic defect the identification of the genetic defect of hereditary of hereditary hemochromatosis prompted us to review hemochromatosis prompted us to review this subject this subject and to redefine the diagnostic criteria of and to redefine the diagnostic criteria of iron over- iron overload disorders. load disorders. Evidence and Information sources. The material exam- ined in the present review includes articles published Classification of iron overload in the journals covered by the Science Citation Index® Iron overload is the result of many disorders and ® and Medline . The author has been working in the field could lead per se to the development of organ dam- of iron overload diseases for several years and has contributed ten of the papers cited in the references. age and increased mortality. In humans total body iron stores is maintained normally within the range State of the art and Perpectives. Iron overload can be of 200-1500 mg (in men, the normal concentration classified on the basis of different criteria: route of of iron in the storage pool is 13 mg/Kg and in access of iron within the organism, predominant tis- sue site of iron accumulation and cause of the over- women 5 mg/kg) by adequate adjustment of intesti- load. -

View Presentation Slides
Visualising the Environment & the Politics of Representation Dr. Joanna Boehnert Center for Science & Technology Policy Research Cooperative Institute for Research in Environmental Sciences University of Colorado, Boulder, USA EcoLabs | www.eco-labs.org | @ecolabs JZ1122 The Earth’s ability to provide an accommodating environment is undermined by our activities. The Earth is our life-supporting system. Despite this basic fact, measured in biophysical terms, the planet is shrinking due to human interventions. Over the past forty years the Living Planet Index (an indicator of the state of biodiversity) has fallen by 30% in northern countries and fallen by 60% in the tropics. During this time there has been a doubling of demands on natural systems. Assessing the capacity of the ecological system to continue to provide THE BALANCE SHEET FOR favorable conditions for civilization must be part GROSS GLOBAL PROSPERITY of an audit of development. Biodiversity has been fallen by a rate of 30% in northern countries and 60% in the tropical world over the past 40 years. PLANETARY BOUNDARIES BIODIVERSITY LOSS NITROGEN FLOW PHOSPHORUS FLOW CLIMATE CHANGE OZONE DEPLETION ATMOSPHERIC AEROSOL LOAD OCEAN ACIDITY FRESHWATER CONSUMPTION CHEMICAL POLLUTION AGRICULTURAL LAND USE Ecological systems have thresholds that can characterized by dynamics where our industrial lead to sudden collapse. Nine planetary patterns are a force dramatically effecting boundaries are central to avoid crossing critical natural, biophysical and geological processes. tipping points. Three boundaries have already The Earth is the foundation for substance, but been transgressed: climate change, the rate of an ecological audit indicates that the model of 97-98% of scientists agree climate 2/3 ecosystems are exploited biodiversity loss and the global nitrogen cycle. -
Genetic Disorders Associated with Metal Metabolism
cells Review Genetic Disorders Associated with Metal Metabolism Muhammad Umair 1 and Majid Alfadhel 1,2,* 1 Medical Genomics Research Department, King Abdullah International Medical Research Center (KAIMRC), King Saud Bin Abdulaziz University for Health Sciences, Ministry of National Guard Health Affairs (MNGH), P.O Box 3660, Riyadh 11481, Saudi Arabia; [email protected] 2 Division of Genetics, Department of Pediatrics, King Abdullah Specialized Children’s Hospital, King Abdulaziz Medical P.O Box 22490, Riyadh 11426, Saudi Arabia * Correspondence: [email protected]; Tel.: +(966)-11-805-3560; Fax: +(966)-11-805-5555 Received: 17 October 2019; Accepted: 5 December 2019; Published: 9 December 2019 Abstract: Genetic disorders associated with metal metabolism form a large group of disorders and mostly result from defects in the proteins/enzymes involved in nutrient metabolism and energy production. These defects can affect different metabolic pathways and cause mild to severe disorders related to metal metabolism. Some disorders have moderate to severe clinical consequences. In severe cases, these elements accumulate in different tissues and organs, particularly the brain. As they are toxic and interfere with normal biological functions, the severity of the disorder increases. However, the human body requires a very small amount of these elements, and a deficiency of or increase in these elements can cause different genetic disorders to occur. Some of the metals discussed in the present review are copper, iron, manganese, zinc, and selenium. These elements may play a key role in the pathology and physiology of the nervous system. Keywords: metal metabolism; genetic disorders; copper; iron; manganese; zinc; selenium 1. Introduction Metal ions play an important role in several biological processes that have both structural and functional importance.