Calcium – ORAL 2019 Newborn Use Only
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Drug Consumption in 2017 - 2020
Page 1 Drug consumption in 2017 - 2020 2020 2019 2018 2017 DDD/ DDD/ DDD/ DDD/ 1000 inhab./ Hospital 1000 inhab./ Hospital 1000 inhab./ Hospital 1000 inhab./ Hospital ATC code Subgroup or chemical substance day % day % day % day % A ALIMENTARY TRACT AND METABOLISM 322,79 3 312,53 4 303,08 4 298,95 4 A01 STOMATOLOGICAL PREPARATIONS 14,28 4 12,82 4 10,77 6 10,46 7 A01A STOMATOLOGICAL PREPARATIONS 14,28 4 12,82 4 10,77 6 10,46 7 A01AA Caries prophylactic agents 11,90 3 10,48 4 8,42 5 8,45 7 A01AA01 sodium fluoride 11,90 3 10,48 4 8,42 5 8,45 7 A01AA03 olaflur 0,00 - 0,00 - 0,00 - 0,00 - A01AB Antiinfectives for local oral treatment 2,36 8 2,31 7 2,31 7 2,02 7 A01AB03 chlorhexidine 2,02 6 2,10 7 2,09 7 1,78 7 A01AB11 various 0,33 21 0,21 0 0,22 0 0,24 0 A01AD Other agents for local oral treatment 0,02 0 0,03 0 0,04 0 - - A01AD02 benzydamine 0,02 0 0,03 0 0,04 0 - - A02 DRUGS FOR ACID RELATED DISORDERS 73,05 3 71,13 3 69,32 3 68,35 3 A02A ANTACIDS 2,23 1 2,22 1 2,20 1 2,30 1 A02AA Magnesium compounds 0,07 22 0,07 22 0,08 22 0,10 19 A02AA04 magnesium hydroxide 0,07 22 0,07 22 0,08 22 0,10 19 A02AD Combinations and complexes of aluminium, 2,17 0 2,15 0 2,12 0 2,20 0 calcium and magnesium compounds A02AD01 ordinary salt combinations 2,17 0 2,15 0 2,12 0 2,20 0 A02B DRUGS FOR PEPTIC ULCER AND 70,82 3 68,91 3 67,12 3 66,05 4 GASTRO-OESOPHAGEAL REFLUX DISEASE (GORD) A02BA H2-receptor antagonists 0,17 7 0,74 4 1,10 4 1,11 5 A02BA02 ranitidine 0,00 1 0,63 3 0,99 3 0,99 4 A02BA03 famotidine 0,16 7 0,11 8 0,11 10 0,12 9 A02BB Prostaglandins 0,04 62 -

Dietary Supplements Compendium Volume 1
2015 Dietary Supplements Compendium DSC Volume 1 General Notices and Requirements USP–NF General Chapters USP–NF Dietary Supplement Monographs USP–NF Excipient Monographs FCC General Provisions FCC Monographs FCC Identity Standards FCC Appendices Reagents, Indicators, and Solutions Reference Tables DSC217M_DSCVol1_Title_2015-01_V3.indd 1 2/2/15 12:18 PM 2 Notice and Warning Concerning U.S. Patent or Trademark Rights The inclusion in the USP Dietary Supplements Compendium of a monograph on any dietary supplement in respect to which patent or trademark rights may exist shall not be deemed, and is not intended as, a grant of, or authority to exercise, any right or privilege protected by such patent or trademark. All such rights and privileges are vested in the patent or trademark owner, and no other person may exercise the same without express permission, authority, or license secured from such patent or trademark owner. Concerning Use of the USP Dietary Supplements Compendium Attention is called to the fact that USP Dietary Supplements Compendium text is fully copyrighted. Authors and others wishing to use portions of the text should request permission to do so from the Legal Department of the United States Pharmacopeial Convention. Copyright © 2015 The United States Pharmacopeial Convention ISBN: 978-1-936424-41-2 12601 Twinbrook Parkway, Rockville, MD 20852 All rights reserved. DSC Contents iii Contents USP Dietary Supplements Compendium Volume 1 Volume 2 Members . v. Preface . v Mission and Preface . 1 Dietary Supplements Admission Evaluations . 1. General Notices and Requirements . 9 USP Dietary Supplement Verification Program . .205 USP–NF General Chapters . 25 Dietary Supplements Regulatory USP–NF Dietary Supplement Monographs . -

Pharmaceutical Appendix to the Tariff Schedule 2
Harmonized Tariff Schedule of the United States (2007) (Rev. 2) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE Harmonized Tariff Schedule of the United States (2007) (Rev. 2) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 2 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. ABACAVIR 136470-78-5 ACIDUM LIDADRONICUM 63132-38-7 ABAFUNGIN 129639-79-8 ACIDUM SALCAPROZICUM 183990-46-7 ABAMECTIN 65195-55-3 ACIDUM SALCLOBUZICUM 387825-03-8 ABANOQUIL 90402-40-7 ACIFRAN 72420-38-3 ABAPERIDONUM 183849-43-6 ACIPIMOX 51037-30-0 ABARELIX 183552-38-7 ACITAZANOLAST 114607-46-4 ABATACEPTUM 332348-12-6 ACITEMATE 101197-99-3 ABCIXIMAB 143653-53-6 ACITRETIN 55079-83-9 ABECARNIL 111841-85-1 ACIVICIN 42228-92-2 ABETIMUSUM 167362-48-3 ACLANTATE 39633-62-0 ABIRATERONE 154229-19-3 ACLARUBICIN 57576-44-0 ABITESARTAN 137882-98-5 ACLATONIUM NAPADISILATE 55077-30-0 ABLUKAST 96566-25-5 ACODAZOLE 79152-85-5 ABRINEURINUM 178535-93-8 ACOLBIFENUM 182167-02-8 ABUNIDAZOLE 91017-58-2 ACONIAZIDE 13410-86-1 ACADESINE 2627-69-2 ACOTIAMIDUM 185106-16-5 ACAMPROSATE 77337-76-9 -

Australian Statistics on Medicines 1997 Commonwealth Department of Health and Family Services
Australian Statistics on Medicines 1997 Commonwealth Department of Health and Family Services Australian Statistics on Medicines 1997 i © Commonwealth of Australia 1998 ISBN 0 642 36772 8 This work is copyright. Apart from any use as permitted under the Copyright Act 1968, no part may be repoduced by any process without written permission from AusInfo. Requests and enquiries concerning reproduction and rights should be directed to the Manager, Legislative Services, AusInfo, GPO Box 1920, Canberra, ACT 2601. Publication approval number 2446 ii FOREWORD The Australian Statistics on Medicines (ASM) is an annual publication produced by the Drug Utilisation Sub-Committee (DUSC) of the Pharmaceutical Benefits Advisory Committee. Comprehensive drug utilisation data are required for a number of purposes including pharmacosurveillance and the targeting and evaluation of quality use of medicines initiatives. It is also needed by regulatory and financing authorities and by the Pharmaceutical Industry. A major aim of the ASM has been to put comprehensive and valid statistics on the Australian use of medicines in the public domain to allow access by all interested parties. Publication of the Australian data facilitates international comparisons of drug utilisation profiles, and encourages international collaboration on drug utilisation research particularly in relation to enhancing the quality use of medicines and health outcomes. The data available in the ASM represent estimates of the aggregate community use (non public hospital) of prescription medicines in Australia. In 1997 the estimated number of prescriptions dispensed through community pharmacies was 179 million prescriptions, a level of increase over 1996 of only 0.4% which was less than the increase in population (1.2%). -

Australian Statistics on Medicines 1999–2000
Commonwealth Department of Health and Ageing Australian Statistics on Medicines 1999–2000 © Commonwealth of Australia 2003 ISBN 0 642 82184 4 This work is copyright. Apart from any use as permitted under the Copyright Act 1968, no part may be reproduced by any process without prior written permission from the Commonwealth available from AusInfo. Requests and inquiries concerning reproduction and rights should be addressed to the Manager, Legislative Services, AusInfo, GPO Box 1920, Canberra ACT 2601. Publications Approval Number: 3183 (PA7270) FOREWORD Comprehensive and valid statistics on use of medicines by Australians in the public domain should be accessible to all interested parties. From the first edition in 1992 until 1999 the Drug Utilisation SubCommittee (DUSC) produced the Australian Statistics on Medicines (ASM) for each calendar year to 1998. It is pleasing indeed to be able to present these again this year, with the inclusion of estimates for the years since the last edition. A continuous data set representing estimates of the aggregate community use (non public hospital) of prescription medicines in Australia is a key tool for the Australian Medicines Policy. The ASM presents dispensing data on most drugs marketed in Australia and is the only current source of data in Australia to cover all prescription medicines dispensed in the community. Drug utilisation data can assist the targeting and evaluation of quality use of medicines initiatives, and the evaluation of changes to the availability of medicines. It is also needed for pharmacosurveillance by regulatory and financing authorities and by the Pharmaceutical Industry. Publication of the Australian data also facilitates international comparisons of drug utilisation profiles and encourages international collaboration on drug utilisation research particularly in relation to enhancing the quality use of medicines and health outcomes. -

List of Annexures Annexure – I Plant Layout Annexure – II Manufacturing Process Annexure – III Details of Storage of So
List of Annexures Annexure – I Plant Layout Annexure – II Manufacturing Process Annexure – III Details of Storage of Solvents Annexure – IV Water Balance & Details of Treatment and disposal of Effluent Annexure – V Details of Treatment and Disposal of Solid Waste Annexure – VI List of Raw materials & Quantity of Consumption Annexure – VII Point Source Emissions – Stack Emission Characteristics Annexure – VIII Details of Fugitive Emissions Annexure – IX Risk Identification Annexure – X Topo Map & Administrative Set-up Map Annexures 1 GCPL ANNEXURE – I PLANT LAYOUT Annexures 2 GCPL Annexures 3 GCPL Annexures 4 GCPL ANNEXURE – II MANUFACTURING PROCESS Annexures 5 GCPL Manufacturing Process Existin g Calcium Gluconate Gluconic Acid procured from market is neutralized with Calcium carbonate, filtered, crystallised, centrifuged, dried, milled and packed. NEUTRALISATION FILTERATION CRYSTALLISATION PACKING MILLING DRYING CENTRIFUGING Alternate process for the manufacture of Calcium Gluconate Gluconic acid is neutralised with Calcium carbonate, filtered, crystallised, centrifuged, dried, milled and packed. NEUTRALISATION FILTRATION CRYSTALLISATION PACKING MILLING DRYING CENTRIFUGING Alternate process GLUCONO DELTA LACTONE + CaCO + WATER 3 FILTRATION CRYSTALLISATION (NEUTRALISATION) PACKING MILLING DRYING CENTRIFUGING Annexures 6 GCPL Sodium Gluconate Gluconic acid brought from the market is neutralised with Sodium bi-carbonate, filtered, concentrated, crystallised, centrifuged, dried, milled and packed. CONCENTRATION NEUTRALISATION FILTRATION CRYSTALLISATION -
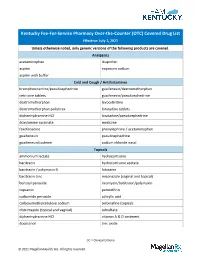
OTC) Covered Drug List Effective: July 1, 2021 Unless Otherwise Noted, Only Generic Versions of the Following Products Are Covered
Kentucky Fee-For-Service Pharmacy Over-the-Counter (OTC) Covered Drug List Effective: July 1, 2021 Unless otherwise noted, only generic versions of the following products are covered. Analgesics acetaminophen ibuprofen aspirin naproxen sodium aspirin with buffer Cold and Cough / Antihistamines brompheniramine/pseudoephedrine guaifenesin/dextromethorphan cetirizine tablets guaifenesin/pseudoephedrine dextromethorphan levocetirizine dextromethorphan polistirex loratadine tablets diphenhydramine HCl loratadine/pseudoephedrine doxylamine succinate meclizine fexofenadine phenylephrine / acetaminophen guaifenesin pseudoephedrine guaifenesin/codeine sodium chloride nasal Topicals ammonium lactate hydrocortisone bacitracin hydrocortisone acetate bacitracin / polymyxin B lidocaine bacitracin zinc miconazole (vaginal and topical) benzoyl peroxide neomycin/bacitracin/polymyxin capsaicin permethrin carbamide peroxide salicylic acid carboxymethylcellulose sodium terbinafine (topical) clotrimazole (topical and vaginal) tolnaftate diphenhydramine HCl vitamin A & D ointment docosanol zinc oxide CC = Clinical Criteria © 2021 Magellan Health, Inc. All rights reserved. Vitamins ascorbic acid (Vitamin C) ferrous sulfate calcium acetate fish oil calcium carbonate / vitamin D3 folic acid calcium citrate magnesium oxide calcium citrate / vitamin D3 niacin /niacinamide cyanocobalamin (Vitamin B-12) prenatal vitamins CC ergocalciferol (vitamin D2) pyridoxine HCl (vitamin B6) ferrous gluconate vitamin D3 GI Products aluminum hydroxide magnesium carbonate / aluminum -

CALCIUM-SANDOZ® Novartis Consumer Health Calcium Preparations Calcium Therapy Indications and Clinical Uses: As a Dietary Suppl
CALCIUM-SANDOZ® Novartis Consumer Health Calcium Preparations Calcium Therapy Indications And Clinical Uses: As a dietary supplement where calcium intake may be inadequate: childhood and adolescence, pregnancy and lactation, postmenopausal females and in the aged. In the treatment of calcium deficiency states which may occur in diseases such as: tetany of the newborn (as a supplement to parenterally administered calcium), hypoparathyroidism (acute and chronic), pseudo-hypoparathyroidism, postmenopausal and senile osteoporosis, rickets and osteomalacia. Contra-Indications: Hypercalcemia and hypercalciuria (e.g. hyperparathyroidism, vitamin D overdosage, decalcifying tumors such as plasmocytoma, bone metastases); severe renal disease; and in calcium loss due to immobilization. Hypersensitivity to any of the components of Calcium-Sandoz. Although no concrete evidence is available about the metabolic process leading from calcium glucono-galacto-gluconate to galactose, it is advisable not to administer to galactosemic patients. Precautions: For diabetic patients, consideration should be given to the sucrose content of 719 mg/tablet of Calcium-Sandoz Forte. Gramcal and Calcium-Sandoz Syrup are suitable for sodium and potassium restricted diets. Calcium-Sandoz Forte is suitable for potassium restricted diets. Because of the presence of sodium (12 mmol), Calcium-Sandoz Forte is not suitable for sodium restricted diets. In mild hypercalciuria (exceeding 300 mg/24 hours) as well as in chronic renal failure, or where there is evidence of stone formation in the urinary tract, adequate checks must be kept on urinary calcium excretion. If necessary the dosage should be reduced or calcium therapy discontinued. In patients prone to formation of calculi in the urinary tract an increased fluid intake is recommended. -

Calcium Supplementation in Pregnant Women
WORLD HEALTH ORGANIZATION DEPARTMENT OF NUTRITION FOR HEALTH AND DEVELOPMENT EVIDENCE AND PROGRAMME GUIDANCE UNIT Calcium supplementation in pregnant women This submission was prepared by Dr Luz Maria De-Regil with technical input from Dr Matthews Mathai, Dr Juan Pablo Pena-Rosas and Harinder Chahal. EML Section 27 – Vitamins and Minerals Table of contents Acronyms and abbreviations........................................................................................................... 2 Executive summary......................................................................................................................... 3 I. Background and rationale for the application.......................................................................... 4 II. Background on calcium and gestation ................................................................................. 4 1. Public health relevance ........................................................................................................ 4 2. Current public health interventions...................................................................................... 5 3. Proposed public health intervention..................................................................................... 5 III. Methods................................................................................................................................ 5 1. Methods for the assessment of dosing, efficacy and safety ................................................. 5 3. Methods for the assessment of -

Copy of Non-Drug Product List Effective 11-15-13
Non-Drug Product List Effective November 15, 2013 Non-Drug Products Indicates Prior Authorization Required Ammonium Lactate Lotion 12% Aquadeks Capsules AquaADEKS Tabs AquaADEKS Solution Bacteriostatic Sodium Chloride Injection 0.9% Bacteriostatic Water for Injection Bacteriostatic Parabens Water for Injection Calcium Carbonate 500mg Chewable Tablets Calcium Carbonate 750mg Chewable Tablets Calcium Carbonate 1000mg Chewable Tablets Calcium Carbonate 1250mg Chewable Tablets Calcium Carbonate 1250mg/5ml Suspension Calcium Carbonate 600mg Tablets Calcium Carbonate-Vitamin D 500mg/200 unit Calcium Carbonate Vitamin D 600mg/200 unit Calcium Carbonate Vitamin D 600mg/400 unit Tablets Calcium Citrate 950mg Tablets Calcium Glubionate 1.8gm/5mL Calcium Gluconate 650mg Tablets Calcium Lactate 650mg Tablets Calvite P&D Tablets Cerovite Jr Chewable Tablets Cerovite Liquid Cholecalciferol 400 Unit Tablets Epoprostenolol Diluents (Flolan) 0.5mg Epoprostenolol Diluents (Flolan) 1.5mg Ferrous Fumarate 325mg Tablets Ferrous Gluconate 324mg Tablets Ferrous Gluconate 325mg Tablets Ferrous Sulfate 75mg/0.6ml Drops Ferrous Sulfate 75mg/ml Drops Ferrous Sulfate 220mg/5ml Elixir Ferrous Sulfate 325mg Tablets Magnesium Chloride ER Tablet 535 (64mg) MG Magnesium Gluconate 1000mg/5mL Magnesium Oxide 400mg Tablets Maximum D3 Capsules Metronidazole Powder Multiple Vitamins Tablets Nephro-vite Tablets Niacin Tablets, 50mg Niacin Tablets, 100mg Niacin Tablets, 250mg Niacin Tablets, 500mg Pediatric Oral Electrolyte Solutions Phos-Nak Powder -

Mainecare Medicare Part D Excluded Drugs Eligible for Coverage Under Mainecare
MaineCare Medicare Part D Excluded Drugs Eligible for Coverage under MaineCare These drugs will maintain the current MaineCare Formulary with enforcement of its existing edits: PDL, Dosage Limitations, etc. Drug Categories / Drug Description Exclusion Cat Drug Categories / Drug Description Exclusion Cat ANALGESICS - MISC. Antacids - Aluminum Salts Salicylates Aluminum Hydroxide Gel OTC Aspirin OTC Antacids - Bicarbonate Analgesics Other Sodium Bicarbonate (Antacid) OTC Acetaminophen OTC Antacids - Calcium Salts ANTIASTHMATIC - NASAL MISC. Calcium Carbonate (Antacid) OTC Nasal Agents - Misc. Antacids - Magnesium Salts Saline OTC ANTICONVULSANTS Magnesium Oxide OTC Anticonvulsants - Benzodiazepines Antacid Combinations Clonazepam Benzodiazepines Aluminum & Magnesium Hydroxide OTC Diazepam (Anticonvulsant) Benzodiazepines GI - ANTIPERISTALTIC AGENTS ANTIHISTAMINES - NON-SEDATING Antiperistaltic Agents Antihistamines - Non-Sedating Loperamide HCl OTC CLARITIN OTC GI - DIGESTIVE ENZYMES loratadine OTC Digestive Enzymes ANXIOLYTICS - BENZODIAZEPINES Lactase OTC Benzodiazepines GI - H2-ANTAGONISTS Alprazolam Benzodiazepines Chlordiazepoxide HCl Benzodiazepines H-2 Antagonists Clorazepate Dipotassium Benzodiazepines Cimetidine OTC Diazepam Benzodiazepines Famotidine OTC Lorazepam Benzodiazepines Ranitidine HCl OTC Oxazepam Benzodiazepines GI - MISC. COUGH/COLD Saline Laxatives Systemic Decongestants Magnesium Citrate OTC Pseudoephedrine HCl OTC Magnesium Hydroxide OTC Topical Decongestants Saline Laxative Mixtures Oxymetazoline HCl OTC FLEET -

Diseases of Bearded Dragons Thomas H
Diseases of Bearded Dragons Thomas H. Boyer, DVM, DABVP (Reptile & Amphibian Practice) Pet Hospital of Penasquitos, San Diego, CA, USA Beardies are the most popular lizard right now. They are prone to their own peculiar problems, the object of this lecture will be learning how to diagnose and treat them, as well as keep them healthy. From feeding, to hibernation, to the many diseases and problems they develop, you will become a bearded dragon expert! Captive Care Bearded dragons are Agamids belonging to the genus Pogona (formerly Amphibolurus) which has multiple species. They are diurnal and terrestrial to semi-arboreal, spending most of their time in bushes and trees, basking on rocks and escaping the heat with underground burrows Bearded dragons arm wave to show submission to avoid aggression, and a head-bobbing to show dominance over other males and signal females they would like to mate with them. Their beard becomes blacker during rivalry between males, but can also turn black to absorb heat. Adults weigh between 350 to 600 g; males grow up to 16 to 24 in, females up to 12 to 20 in long. In the wild bearded dragons are solitary. In captivity males will fight with other males and should be housed singly, or in groups of two or more females. Juveniles can be raised in screened 10 to 20 gallons aquariums, adults need 50 gallon aquariums, minimum, or larger. Newspaper, paper towels, or paper pulp substrate work well. Sand, gravel and many other substrates can cause colonic impaction. Climbing branches, cork bark hides, basking rocks and burrowing areas, such as a humid retreat, should be available.