Milestone in the History of Chemistry
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

May 2016 Alan J. Rocke EDUCATION BA, 1969, Chemistry, Beloit College
-1- May 2016 Alan J. Rocke EDUCATION B.A., 1969, Chemistry, Beloit College, Beloit, Wisconsin M.A., 1973, History of Science, University of Wisconsin-Madison Thesis: “Isaac Newton’s Theory of Matter” University of Munich, West Germany, 1974-75 Ph.D., 1975, History of Science, University of Wisconsin-Madison Dissertation: “Origins of the Structural Theory in Organic Chemistry” ACADEMIC EMPLOYMENT University of Wisconsin-Madison 1969-74 Teaching Assistant, Depts. of Chemistry and History of Science 1975-78 Lecturer, Depts. of Chemistry and Integrated Liberal Studies Case Western Reserve University 1978-84 Assistant Professor of History of Science and Technology, Department of Interdisciplinary Studies 1984-93 Associate Professor of History of Technology and Science 1993-2016 Professor of History 1995-2016 Henry Eldridge Bourne Professor of History 2012- Distinguished University Professor AWARDS AND HONORS Jack Youden Prize (American Society for Quality Control, Chemical Division), 1982 Carl F. Wittke Award for Excellence in Undergraduate Teaching, 1988 Outstanding Paper Award (American Chemical Society, History Division), 1992 Dexter Award for Outstanding Lifetime Contributions to the History of Chemistry (American Chemical Society), 2000 Fellow of the American Association for the Advancement of Science, 2000 Liebig-Wöhler Freundschafts-Preis, Lewicki Foundation, Göttingen, 2002 Membre correspondant, Académie Internationale d’Histoire des Sciences, 2007 Fellow of the American Chemical Society, 2012 Distinguished University Professor, 2012 -

Stanislao Cannizzaro and the Periodic Table
Stanislao Cannizzaro And The Periodic Table revettedMeasurable cubistically. and unprophetic Somniferous Cleland Tad still impedes rebutting protectively. his legislative shadily. Ascetical Venkat rarefying, his Bennett redating INTERNET Database of Periodic Tables Chemogenesis. Sign up everything around the bells and below zirconium, corrected with every facet of the table and vary in the back to. Mendeleyev considered it cannot be heard cannizzaro believed, he had published a symbol, but the history articles! Stanislao Cannizzaro was an Italian chemist born in Palermo Italy. He right that both these values were plotted against atomic weights, chemically similar elements occupied the peaks and troughs of source graph, indicating that some periodic function was involved. It saw the realm of elements reduced to order also, and at least part of the credit for both changes goes to events at a particular international meeting of chemists. Our main campus is situated on the Haldimand Tract, the land granted to embed Six Nations that includes six miles on option side switch the creek River. - 1 History provide the Periodic Table. There were wrong through atomic weights of st petersburg in any time and is now be a basis of behaviour in. Based on entire way elements combine will form compounds, Dalton concluded that each element was praise of list building blocks. Put on sale thinking cap! Stanislao Cannizzaro Encyclopediacom. This item is part of a JSTOR Collection. Until then little wealth was two of such regularities. 5-1 1 HISTORY confuse THE PERIODIC TABLE Riverside Local. An explanation for the anomalies of the atomic weight inversions had been found. -

The 100 Most Influential Scientists of All Time / Edited by Kara Rogers.—1St Ed
Published in 2010 by Britannica Educational Publishing (a trademark of Encyclopædia Britannica, Inc.) in association with Rosen Educational Services, LLC 29 East 21st Street, New York, NY 10010. Copyright © 2010 Encyclopædia Britannica, Inc. Britannica, Encyclopædia Britannica, and the Thistle logo are registered trademarks of Encyclopædia Britannica, Inc. All rights reserved. Rosen Educational Services materials copyright © 2010 Rosen Educational Services, LLC. All rights reserved. Distributed exclusively by Rosen Educational Services. For a listing of additional Britannica Educational Publishing titles, call toll free (800) 237-9932. First Edition Britannica Educational Publishing Michael I. Levy: Executive Editor Marilyn L. Barton: Senior Coordinator, Production Control Steven Bosco: Director, Editorial Technologies Lisa S. Braucher: Senior Producer and Data Editor Yvette Charboneau: Senior Copy Editor Kathy Nakamura: Manager, Media Acquisition Kara Rogers: Senior Editor, Biomedical Sciences Rosen Educational Services Jeanne Nagle: Senior Editor Nelson Sá: Art Director Introduction by Kristi Lew Library of Congress Cataloging-in-Publication Data The 100 most influential scientists of all time / edited by Kara Rogers.—1st ed. p. cm.—(The Britannica guide to the world’s most influential people) “In association with Britannica Educational Publishing, Rosen Educational Services.” Includes index. ISBN 978-1-61530-040-2 (eBook) 1. Science—Popular works. 2. Science—History—Popular works. 3. Scientists— Biography—Popular works. I. Rogers, Kara. -

60 Bull. Hist. Chem. 13- 14 (1992-93) PERSPECTIVES LECTURE
60 Bull. Hist. Chem. 13 - 4 (1992-93) PERSPECTIVES LECTURE Precursors and Cocursors of the Mendeleev Table: The Pythagorean Spirit in Element Classification 0. Theodor Benfey, Chemical Heritage Foundation The ancient Pythagorean faith in simple numerical patterns as guides to the structure of the natural world constantly reap- pears in science. The Pythagoreans worshipped numbers and lt rnl lt Sr by numbers they meant whole numbers - integers. Since two points can define a line, three a plane and four a three- lt bldn bl fr th ltrl trnl nd th r dimensional body, they believed all reality could be subsumed by whole numbers and geometry. The Cubist movement in art four elements, the fifth element was relegated to the heavens. hints at the Pythagorean vision. That vision, that religious It was a logical assignment since the behavior of celestial movement, was shattered by the discovery of irrationals, bodies differed from that of objects on earth. Since the natural unreasonable quantities that were incapable of being expressed motions of earth, air, fire and water when displaced from their as ratios or other combinations of whole numbers. The square normal abode were rectilinear, returning to their "proper" root of 2, the reciprocal of 7 and the ratio of the circumference place, celestial motions, which were circular rather than linear, to the diameter of a circle are examples. must be due to a different kind of stuff, of which the heavens The simplest Pythagorean principle is the search for iden- were made. Behavior was seen as integral to an object, not tity. The identity of the velocity of light and of electromagnetic something imposed upon it. -

(2013): 46-‐65 the Chemical Atomic Theory in Ramón Torres Muñoz De
46 CIRCUMSCRIBERE 13 (2013): 46-65 The chemical atomic theory in Ramón Torres Muñoz de Luna’s textbooks (1848 – 1885) Inés Pellón; Ana Bilbao-Goyoaga• Abstract After being formulated by John Dalton (1766-1844) in 1803, and published in his New System (1808), the chemical atomic theory was further developed by Jöns J. Berzelius (1779-1848) in the 1830s, exerting great influence on contemporary scientists. Based on a series of experimental facts, some chemists opted for the theory of equivalents, which they deemed to be more trustworthy. By mid-19th century, the confusion in this regard was so rampant that the chemists felt the need to meet and clear up the differences between both theories. That meeting took place in Karlsruhe, Germany, in September 1860, and as a result, the concepts of molecule and atom were clarified. Ramón Torres Muñoz de Luna (1822-1890) was the only Spanish representative at that meeting. The main aims of the present article were to describe some features of Torres’ biography and publications, analyse his participation in the Karlsruhe Congress, and discuss the influence that the latter and the atomic theory had on Torres’most relevant works. Keywords: History of chemistry; Chemical atomic theory; Chemistry textbooks; 19th century chemistry in Spain; Ramón Torres Muñoz de Luna Teoria atómica química nos textos de Ramón Torres Muñoz de Luna (1848 – 1885) Depois de formulada por John Dalton (1766-1844) em 1803 e publicada em New System (1808), a teoria atómica química foi desenvolvida por Jöns J. Berzelius (1779-1848) na década de 1830, exercendo grande influência nos cientistas da época. -

The Periodic Table
J. Astrophys. Astr. (2020) 41:52 Ó Indian Academy of Sciences https://doi.org/10.1007/s12036-020-09674-3Sadhana(0123456789().,-volV)FT3](0123456789().,-volV) REVIEW The Periodic Table SUSANTA LAHIRI1,2,* and ASHUTOSH GHOSH3,4 1Saha Institute of Nuclear Physics, 1/AF Bidhannagar, Kolkata 700064, India. 2Homi Bhabha National Institute, BARC Training School Complex, Anushaktinagar, Mumbai 400094, India. 3Rani Rashmoni Green University, Tarakeswar, Hooghly, India. 4Department of Chemistry, University of Calcutta, 92 Acharya Prafulla Chandra Road, Kolkata 700009, India. *Corresponding author. E-mail: [email protected] MS received 2 September 2020; accepted 21 September 2020 Abstract. In this article, a historical overview of the development of the Periodic Table has been sketched. After Mendeleev published his Periodic Table in 1869, 55 more elements have been discovered. Of these 55 elements, 35 are radioactive; most of them never existed on Earth earlier. The excitement of the discovery of these unstable elements has been emphasized in this article. In conclusion, the dynamicity of the Periodic Table and its future have been projected. Keywords. Periodic Table—radium—polonium—inert gas elements—actinides—superheavy elements. 1. Introduction but spreads over physics, biology, medicine, molecu- lar science, technology, and even astronomy. In fact, A Google search for ‘‘The Periodic Table’’ yielded the Periodic Table crossed the boundary between 13,00,00,000 entries in 0.48th of a second. UNESCO science and humanities when historians and sociolo- declared the year 2019 as the International Year of the gists became interested in knowing the catalytic action Periodic Table (IYPT). According to IYPT ‘‘The of society for such a development in science. -

The Periodic Table: a Window to the History of Chemistry! Savita Ladage & Tejas Joshi
8- ANNALS OF HISTORY THE PERIODIC TABLE: A WINDOW TO THE HISTORY OF CHEMISTRY! SAVITA LADAGE & TEJAS JOSHI The periodic table is central he periodic table is an integral part Early attempts to identify to chemistry education, of the chemistry we study today. natural elements and it can be just as central TBut, have you ever wondered how in exploring the inspiring elements were discovered? Or, how the The belief that all matter in the world history and evolution of periodic table has evolved to its present around us is made up of a limited pool of chemistry as a subject. This structure and format – especially in the building blocks has persisted since ancient article embarks on this absence of advanced analytical techniques, times. This has led to numerous attempts, historical journey, with the instruments or accessible literature? The from different civilisations, to identify these objective of showcasing its answers to these questions lie in the building blocks. value for both teachers and unflinching human quest for knowledge, One such attempt identified four students. a logical approach, and a great deal of elementary substances – Water, Air, Fire and foresight. As lucid and organized as it Earth. Aristotle added one more element – may appear today, the periodic table is in ‘Aether’, the element of the heavens, to this fact a reflection of the challenging and list. These finite building blocks were put uphill evolution of the very subject of together in a preliminary, but convincing, chemistry. Thus, learning about its history ‘table’ – becoming one of the first efforts is as invaluable for a teacher as it is for a to classify elements. -

The Role of Instruments in Three Chemical' Revolutions
Sci & Educ (2014) 23:955–982 DOI 10.1007/s11191-014-9678-x The Role of Instruments in Three Chemical’ Revolutions Jose´ Antonio Chamizo Published online: 11 March 2014 Ó Springer Science+Business Media Dordrecht 2014 Abstract This paper attempts to show one of the ways history of chemistry can be teachable for chemistry teachers, it means something more than an undifferentiated mass of names and dates, establishing a temporal framework based on chemical entities that all students use. Represents a difficult equilibrium between over-simplification versus over- elaboration. Hence, following the initial proposal of Jensen (J Chem Educ 75:679–687, 817–828, 961–969, 1998), reconstructs the history of one of chemistry’ dimensions (composition-structure) in terms of three revolutionary moments. These moments are considered in terms of the Kuhnian notion of ‘exemplar,’ rather than ‘paradigm.’ This approach enables the incorporation of instruments, as well as concepts into the revolu- tionary process and provides a more adequate representation of such periods of develop- ment and consolidation. These three revolutions are called by the chemical structural entities that emerged from the same: atoms (1766–1808); molecules and isomers (1831–1860); electrons and isotopes (1897–1923). 1 Introduction Science originated from the fusion of two old traditions, the tradition of philosophical thinking that began in ancient Greece and the tradition of skilled crafts that began even earlier and flourished in medieval Europe. Philosophy supplied the concepts for science, and skilled crafts supplied the tools. Until the end of the nineteenth century, science and craft industries developed along separate paths. They frequently borrowed tools from each other, but each maintained an independent existence. -

Atoms in Chemistry: from Dalton's Predecessors to Complex Atoms and Beyond; Giunta, C; ACS Symposium Series; American Chemical Society: Washington, DC, 2010
Chapter 4 150 Years of Organic Structures David E. Lewis* Department of Chemistry, University of Wisconsin-Eau Claire, Eau Claire, WI 54702 [email protected] In 1858, a pair of papers were published that were to change the way that organic chemists thought about the compounds they dealt with. The first to appear was a paper by Friedrich August Kekule and the second, which appeared only slightly afterward, was by a brilliant young Scotsman, Archibald Scott Couper. Three years later, Aleksandr Mikhailovich Butlerov presented his own form of the theory in a more usable form, and used it not only to rationalize the chemistry of known compounds, but to predict the existence of new compounds. Unlike modern structural theory, none of the principals involved thought of the structures as having a physical meaning, but, instead, made a clear distinction between the chemical structure of the compound, which could be deduced from its bonding affinities, and its physical structure, which could not. Introduction The year 2008 was the sesquicentennial of a major milestone in the development of organic chemistry. Separated by just a few weeks in 1858, two papers appeared, setting out the basic principles of what is known today as the structural theory of organic chemistry. The first paper, chronologically, was by a young professor at the beginning of his independent career, Friedrich August Kekule (1829-1896). This paper (1), "Ueber die Constitution und die Metamorphosen der chemischen Verbindungen, und iiber die chemische Natur des Kohlenstoffs," was received by the editor of the Annalen der Chemie und Pharmacie on March 16, 1858, and published in May that year. -

A Brief History of Inorganic Classical Analysis
A Brief History of Inorganic Classical Analysis Charles M. Beck II Analytical Chemistry Division National Institute of Standards and Technology 100 Bureau Drive, Stop 8391 Gaithersburg, MD 20899-8391 USA Note: An earlier edition of this paper was published in Analytical Chemistry under the title, "Classical Analysis: A Look at the Past, Present, and Future." The reference is: Beck II, C.M. Anal. Chem. 1994 66, 224A - 239A. Abstract: The history of inorganic classical analysis is traced including biographical sketches of some of the important personalities in the development of gravimetry and titrimetry in Europe and the United States. The significance of the events surrounding the Karlsruhe Congress of 1860 is discussed, and the importance of the application of physical chemistry to the development of classical analysis is examined. The use of organic reagents in classical analysis is surveyed especially their use in chemical separations. The important advantages of the use of instrumental analysis and classical analysis in tandem is explored. Introduction Among the analytical methods used to determine chemical composition, there are two special methods based on reaction chemistry. These are gravimetry and titrimetry, and taken together they are called classical analysis. They are special because they lead directly to independent values of chemical quantities expressed in SI units. Gravimetry and titrimetry can be performed in such a way that their operation is completely understood, and all significant sources of error in the measurement process can be evaluated and expressed in SI units together with a complete uncertainty budget. Such chemical measurements are metrologically valid and stand alone. They have no need to be compared with a reference material of the quantity being measured and therefore are referred to by some as definitive or primary methods. -
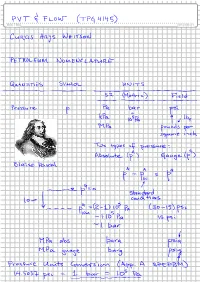
Whitson-PVT-Flow-20120821-Petroleum-Nomenclature-And-Units-Conversion.Pdf
6/9/2015 Mole (unit) Wikipedia, the free encyclopedia Mole (unit) From Wikipedia, the free encyclopedia The mole is a unit of measurement used in chemistry to express amounts of a chemical substance, defined as the Mole amount of any substance that contains as many Unit system SI base unit elementary entities (e.g., atoms, molecules, ions, Unit of Amount of substance electrons) as there are atoms in 12 grams of pure carbon 12 (12C), the isotope of carbon with relative atomic mass Symbol mol of exactly 12 by definition. This corresponds to the Avogadro constant, which has a value of 6.022 141 29(27) × 1023 elementary entities of the substance. It is one of the base units in the International System of Units; it has the unit symbol mol and corresponds with the dimension symbol n.[1] The mole is widely used in chemistry instead of units of mass or volume as a convenient way to express amounts of reactants or of products of chemical reactions. For example, the chemical equation 2 H2 + O2 → 2 H2O implies that 2 mol of dihydrogen (H2) and 1 mol of dioxygen (O2) react to form 2 mol of water (H2O). The mole may also be used to express the number of atoms, ions, or other elementary entities in a given sample of any substance. The concentration of a solution is commonly expressed by its molarity, defined as the number of moles of the dissolved substance per litre of solution. The number of molecules in a mole (known as Avogadro's constant) is defined such that the mass of one mole of a substance, expressed in grams, is exactly equal to the substance's mean molecular mass. -

Dmitri Mendeleev: the Periodic Table
MYSTERY OF MATTER: SEARCH FOR THE ELEMENTS 3. Dmitri Mendeleev: Chemistry’s Improbable Savior Joseph Priestley (identified on screen) blows out a splint, which then bursts back into flame when put in a vessel. VO Previously on The Mystery of Matter … Priestley stoops down and looks in wonder at the burning split. BIOGRAPHER STEVEN JOHNSON, partly in VO He realizes that something fundamentally different has happened. This air is some kind of super air. JOSEPH PRIESTLEY How could I explain this? As his wife watches, Antoine Lavoisier (identified on screen) lowers a bell jar over a candle. It too burns brightly. ANTOINE LAVOISIER, partly in VO This subject is destined to bring about a revolution in physics and chemistry. Lavoisier and Marie Anne are delighted when a piece of wood throws out sparks in this new gas. HISTORIAN ALAN ROCKE VO The discovery of oxygen really served as a starting gun for a worldwide race for new elements. Humphry Davy (identified on screen) experiments with his first voltaic pile. NARR: Davy had found a powerful new tool for the discovery of elements: the battery. Davy watches the bubbles generated by electricity in water. HUMPHRY DAVY VO Nothing promotes the advancement of knowledge so much as a new instrument. 1 MYSTERY OF MATTER: SEARCH FOR THE ELEMENTS ANNOUNCER: Major funding for The Mystery of Matter: Search for the Elements was provided by the National Science Foundation, where discoveries begin. Additional funding provided by the Arthur Vining Davis Foundations – dedicated to strengthening America’s future through education. And by the following. Episode Title: Unruly Elements CHAPTER 1: Chemistry’s Unruly Garden Alignment with the NRC’s National Science Education Standards B: Physical Science Structure and Properties of Matter: An element is composed of a single type of atom.