Solutions (PDF)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

5G Candidate Band Study
5G Candidate Band Study Study on the Suitability of Potential Candidate Frequency Bands above 6GHz for Future 5G Mobile Broadband Systems Final Report to Ofcom, March 2015 Contributors Steve Methley, William Webb, Stuart Walker, John Parker Quotient Associates Limited Compass House, Vision Park, Chivers Way, Histon, Cambridge, CB24 9AD, UK EMAIL [email protected] WEB www.QuotientAssociates.com Ver si on 02 Status Approved History Reviewed by Ofcom Type Public 5G Candidate Band Study | Contributors Final Report : qa1015 © Quotient Associates Ltd. 2015 Commercial in Confidence. No part of the contents of this document may be disclosed, used or reproduced in any form, or by any means, without the prior written consent of Quotient Associates Ltd. ii EXECUTIVE SUMMARY Objective and scope Within industry and academia there is significant activity in research and development towards the next generation of mobile broadband technologies (5G). Coupled with this there is increasing interest in identifying the frequency bands that will be needed to deliver 5G services. At the moment there is no overall consensus on what 5G will actually be. Work in ITU-R (WP5D) is ongoing to develop a vision for 5G (IMT 2020) including identifying the target capabilities. It is, however, widely accepted that at least one element of 5G will require the use of spectrum bands at much higher frequencies than those that current mobile broadband technologies can make use of, e.g. bands above 6 GHz. A first step currently being discussed is a proposal for an agenda item for the World Radio Conference after next (WRC-19) to identify suitable high frequency spectrum for 5G in the ITU Radio Regulations. -

Wide Band Gap Materials: Revolution in Automotive Power Electronics
20169052 16mm Wide Band Gap Materials: Revolution in Automotive Power Electronics Luca Bartolomeo 1) Luigi Abbatelli 2) Michele Macauda 2) Filippo Di Giovanni 2) Giuseppe Catalisano 2) Miroslav Ryzek 3) Daniel Kohout 3) 1) STMicroelectronics Japan, Shinagawa INTERCITY Tower A, 2-15-1, Konan, Minato-ku, 108-6017 Tokyo, Japan (E-mail: [email protected]) 2) STMicroelectronics , Italy 3) STMicroelectronics , Czech Republic ④④④ ④④④ Presented at the EVTeC and APE Japan on May 26, 2016 ABSTRACT : The number of Electric and Hybrid vehicles on the roads is increasing year over year. The role of power electronics is of paramount importance to improve their efficiency, keeping lighter and smaller systems. In this paper, the authors will specifically cover the use of Wide Band Gap (WBG) materials in Electric and Hybrid vehicles. It will be shown how SiC MOSFETs bring significant benefits compared to standard IGBTs silicon technology, in both efficiency and form factor. Comparison of the main electrical characteristics, between SiC-based and IGBT module, were simulated and validated by experimental tests in a real automotive environment. KEY WORDS : SiC, Wide Band Gap, IGBT, Power Module 1. INTRODUCTION When considering power transistors in the high voltage range Nowadays, an increased efficiency demand is required in (above 600V), SiC MOSFETs are an excellent alternative to the power electronics applications to have lighter and smaller standard silicon devices: they guarantee lower RON*Area values systems and to improve the range of new Electric (EV) and compared to the latest silicon-based Super-Junction MOSFETs, Hybrid vehicles (HEV). There is an on-going revolution in the especially in high temperature environment (1). -

Design of SWB Antenna with Triple Band Notch Characteristics for Multipurpose Wireless Applications
applied sciences Article Design of SWB Antenna with Triple Band Notch Characteristics for Multipurpose Wireless Applications Warsha Balani 1, Mrinal Sarvagya 1, Tanweer Ali 2,* , Ajit Samasgikar 3, Saumya Das 4, Pradeep Kumar 5,* and Jaume Anguera 6,7 1 School of Electronics and Communication Engineering, Reva University, Bangalore 560064, India; [email protected] (W.B.); [email protected] (M.S.) 2 Department of Electronics and Communication, Manipal Institute of Technology, Manipal Academy of Higher Education, Manipal, Karnataka 576104, India 3 MMRFIC Technology Pvt Ltd., Bangalore 560016, India; [email protected] 4 Department of Information Technology, Sikkim Manipal Institute of Technology, Sikkim Manipal University, Sikkim 737102, India; [email protected] 5 Discipline of Electrical, Electronic and Computer Engineering, University of KwaZulu-Natal, Durban 4041, South Africa 6 Fractus Antennas, 08174 Barcelona, Spain; [email protected] 7 Electronics and Telecommunication Department, Universitat Ramon LLull, 08022 Barcelona, Spain * Correspondence: [email protected] (T.A.); [email protected] (P.K.) Abstract: A compact concentric structured monopole antenna for super wide band (SWB) applica- tions with triple notch band characteristics is designed and experimentally validated. The antenna covers an immense impedance bandwidth (1.6–47.5 GHz) with sharp triple notch bands at 1.8– 2.2 GHz, 4–7.2 GHz, and 9.8–10.4 GHz to eliminate interference from co-existing advanced wireless services (AWS), C bands, and X bands, respectively. By loading an E-shaped stub connected at the top of the patch and by etching a split elliptical slot at the lower end of the radiating patch, the band rejection characteristics from 1.8–2.2 GHz for the AWS and 4–7.2 GHz for the C band are achieved, Citation: Balani, W.; Sarvagya, M.; respectively. -

Towards Direct-Gap Silicon Phases by the Inverse Band Structure Design Approach
Towards Direct-Gap Silicon Phases by the Inverse Band Structure Design Approach H. J. Xiang1,2*, Bing Huang2, Erjun Kan3, Su-Huai Wei2, X. G. Gong1 1 Key Laboratory of Computational Physical Sciences (Ministry of Education), State Key Laboratory of Surface Physics, and Department of Physics, Fudan University, Shanghai 200433, P. R. China 2National Renewable Energy Laboratory, Golden, Colorado 80401, USA 3Department of Applied Physics, Nanjing University of Science and Technology, Nanjing, Jiangsu 210094, People’s Republic of China e-mail: [email protected] Abstract Diamond silicon (Si) is the leading material in current solar cell market. However, diamond Si is an indirect band gap semiconductor with a large energy difference (2.4 eV) between the direct gap and the indirect gap, which makes it an inefficient absorber of light. In this work, we develop a novel inverse-band structure design approach based on the particle swarming optimization algorithm to predict the metastable Si phases with better optical properties than diamond Si. Using our new method, we predict a cubic Si20 phase with quasi- direct gaps of 1.55 eV, which is a promising candidate for making thin-film solar cells. PACS: 71.20.-b,42.79.Ek,78.20.Ci,88.40.jj 1 Due to the high stability, high abundance, and the existence of an excellent compatible oxide (SiO2), Si is the leading material of microelectronic devices. Currently, the majority of solar cells fabricated to date have also been based on diamond Si in monocrystalline or large- grained polycrystalline form [1]. There are mainly two reasons for this: First, Si is the second most abundant element in the earth's crust; Second, the Si based photovoltaics (PV) industry could benefit from the successful Si based microelectronics industry. -

CSE- Module-3: SEMICONDUCTOR LIGHT EMITTING DIODE :LED (5Lectures)
CSE-Module- 3:LED PHYSICS CSE- Module-3: SEMICONDUCTOR LIGHT EMITTING DIODE :LED (5Lectures) Light Emitting Diodes (LEDs) Light Emitting Diodes (LEDs) are semiconductors p-n junction operating under proper forward biased conditions and are capable of emitting external spontaneous radiations in the visible range (370 nm to 770 nm) or the nearby ultraviolet and infrared regions of the electromagnetic spectrum General Structure LEDs are special diodes that emit light when connected in a circuit. They are frequently used as “pilot light” in electronic appliances in to indicate whether the circuit is closed or not. The structure and circuit symbol is shown in Fig.1. The two wires extending below the LED epoxy enclose or the “bulb” indicate how the LED should be connected into a circuit or not. The negative side of the LED is indicated in two ways (1) by the flat side of the bulb and (2) by the shorter of the two wires extending from the LED. The negative lead should be connected to the negative terminal of a battery. LEDs operate at relative low voltage between 1 and 4 volts, and draw current between 10 and 40 milliamperes. Voltages and Fig.-1 : Structure of LED current substantially above these values can melt a LED chip. The most important part of a light emitting diode (LED) is the semiconductor chip located in the centre of the bulb and is attached to the 1 CSE-Module- 3:LED top of the anvil. The chip has two regions separated by a junction. The p- region is dominated by positive electric charges, and the n-region is dominated by negative electric charges. -

The Band Gap of Silicon
Norton 0 The Band Gap of Silicon Matthew Norton, Erin Stefanik, Ryan Allured, and Drew Sulski Norton 1 Abstract This experiment was designed to find the band gap of silicon as well as the charge of an electron. A transistor was heated to various temperatures using a hot plate. The resistance was measured over the resistor and transistor in the circuit. In performing this experiment, it was found that the band gap of silicon was (1.10 ± 0.08) eV. In performing this experiment, it was also found that the charge of an electron was (1.77 ± 0.20) × 10 −19 C. Introduction When a substance is placed under the influence of an electric field, it can portray insulating, semi-conducting, semi-metallic, or metallic properties. Every crystalline structure has electrons that occupy energy bands. In a semiconductor, there is a gap in energy between valence band and the bottom of the conduction band. There are no allowed energy states for the electron within the energy gap. At absolute zero, all the electrons have energies within the valence band and the material it is insulating. As the temperature increases electrons gain enough energy to occupy the energy levels in the conduction band. The current through a transistor is given by the equation, qV I= I e kT − 1 0 (1) where I0 is the maximum current for a large reverse bias voltage, q is the charge of the electron, k is the Boltzmann constant, and T is the temperature in Kelvin. As long as V is not too large, the current depends only on the number of minority carriers in the conduction band and the rate at which they diffuse. -

Assessment on Use of Spectrum in the 10-17 Ghz Band for the GSO Fixed-Satellite Service in Region 1
Report ITU-R S.2365-0 (09/2015) Assessment on use of spectrum in the 10-17 GHz band for the GSO fixed-satellite service in Region 1 S Series Fixed satellite service ii Rep. ITU-R S.2365-0 Foreword The role of the Radiocommunication Sector is to ensure the rational, equitable, efficient and economical use of the radio- frequency spectrum by all radiocommunication services, including satellite services, and carry out studies without limit of frequency range on the basis of which Recommendations are adopted. The regulatory and policy functions of the Radiocommunication Sector are performed by World and Regional Radiocommunication Conferences and Radiocommunication Assemblies supported by Study Groups. Policy on Intellectual Property Right (IPR) ITU-R policy on IPR is described in the Common Patent Policy for ITU-T/ITU-R/ISO/IEC referenced in Annex 1 of Resolution ITU-R 1. Forms to be used for the submission of patent statements and licensing declarations by patent holders are available from http://www.itu.int/ITU-R/go/patents/en where the Guidelines for Implementation of the Common Patent Policy for ITU-T/ITU-R/ISO/IEC and the ITU-R patent information database can also be found. Series of ITU-R Reports (Also available online at http://www.itu.int/publ/R-REP/en) Series Title BO Satellite delivery BR Recording for production, archival and play-out; film for television BS Broadcasting service (sound) BT Broadcasting service (television) F Fixed service M Mobile, radiodetermination, amateur and related satellite services P Radiowave propagation RA Radio astronomy RS Remote sensing systems S Fixed-satellite service SA Space applications and meteorology SF Frequency sharing and coordination between fixed-satellite and fixed service systems SM Spectrum management Note: This ITU-R Report was approved in English by the Study Group under the procedure detailed in Resolution ITU-R 1. -

Terahertz (Thz) Generator and Detection
Electrical Science & Engineering | Volume 02 | Issue 01 | April 2020 Electrical Science & Engineering https://ojs.bilpublishing.com/index.php/ese REVIEW Terahertz (THz) Generator and Detection Jitao Li#,* Jie Li# School of Precision Instruments and OptoElectronics Engineering, Tianjin University, Tianjin, 300072, China #Authors contribute equally to this work. ARTICLE INFO ABSTRACT Article history In the whole research process of electromagnetic wave, the research of Received: 26 March 2020 terahertz wave belongs to a blank for a long time, which is the least known and least developed by far. But now, people are trying to make up the blank Accepted: 30 March 2020 and develop terahertz better and better. The charm of terahertz wave origi- Published Online: 30 April 2020 nates from its multiple attributes, including electromagnetic field attribute, photon attribute and thermal attribute, which also attracts the attention of Keywords: researchers in different fields and different countries, and also terahertz Terahertz technology have been rated as one of the top ten technologies to change the future world by the United States. The multiple attributes of terahertz make Generation it have broad application prospects in military and civil fields, such as med- Detection ical imaging, astronomical observation, 6G communication, environmental monitoring and material analysis. It is no exaggeration to say that mastering terahertz technology means mastering the future. However, it is because of the multiple attributes of terahertz that the terahertz wave is difficult to be mastered. Although terahertz has been applied in some fields, controlling terahertz (such as generation and detection) is still an important issue. Now- adays, a variety of terahertz generation and detection technologies have been developed and continuously improved. -

Wide Band-Gap Semiconductor Based Power Electronics for Energy Efficiency
Wide Band-Gap Semiconductor Based Power Electronics for Energy Efficiency Isik C. Kizilyalli, Eric P. Carlson, Daniel W. Cunningham, Joseph S. Manser, Yanzhi “Ann” Xu, Alan Y. Liu March 13, 2018 United States Department of Energy Washington, DC 20585 TABLE OF CONTENTS Abstract 2 Introduction 2 Technical Opportunity 2 Application Space 4 Evolution of ARPA-E’s Focused Programs in Power Electronics 6 Broad Exploration of Power Electronics Landscape - ADEPT 7 Solar Photovoltaics Applications – Solar ADEPT 10 Wide-Bandgap Materials and Devices – SWITCHES 11 Addressing Material Challenges - PNDIODES 16 System-Level Advances - CIRCUITS 18 Impacts 21 Conclusions 22 Appendix: ARPA-E Power Electronics Projects 23 ABSTRACT The U.S. Department of Energy’s Advanced Research Project Agency for Energy (ARPA-E) was established in 2009 to fund creative, out-of-the-box, transformational energy technologies that are too early for private-sector investment at make-or break points in their technology development cycle. Development of advanced power electronics with unprecedented functionality, efficiency, reliability, and reduced form factor are required in an increasingly electrified world economy. Fast switching power semiconductor devices are the key to increasing the efficiency and reducing the size of power electronic systems. Recent advances in wide band-gap (WBG) semiconductor materials, such as silicon carbide (SiC) and gallium nitride (GaN) are enabling a new genera- tion of power semiconductor devices that far exceed the performance of silicon-based devices. Past ARPA-E programs (ADEPT, Solar ADEPT, and SWITCHES) have enabled innovations throughout the power electronics value chain, especially in the area of WBG semiconductors. The two recently launched programs by ARPA-E (CIRCUITS and PNDIODES) continue to investigate the use of WBG semiconductors in power electronics. -

The Field-Effect Transistor (FET) in a Field-Effect Transistor, an Applied Voltage Is Used to Change the Conductivity of an Electron Or “Hole” Channel
The Field-Effect Transistor (FET) In a field-effect transistor, an applied voltage is used to change the conductivity of an electron or “hole” channel. To understand the operation of a FET, we first should understand just a little about the chemistry and physics of semiconductor devices. Let us consider, for simplicity, only silicon devices. Some Background (this section is Explore More!...will not be tested) Silicon (Si) is an element of the periodic table that contains 4 electrons in its “outer shell”. Pure silicon can arrange itself in a diamond cubic crystal structure (ref: http://en.wikipedia.org/wiki/Band_gap) where it shares its electrons with other Si atoms. The valance band is nominally full and the conduction band is nominally empty, thus resulting in an insulating material. Within this structure, Si forms an insulating material with a band gap energy of 1.11 electron volts (1.6e-19J, the amount of energy needed to move one electron across a one volt potential) separating the valance band from the conduction band. Figure 1: Electron band gap of an undoped (intrinsic) semiconductor. When doped (or infused) with an element that contains 5 electrons in its outer shell (say, for instance, nitrogen, N), this element will displace one silicon atom. Only using 4 of its 5 outer-shell electrons to form tight bonds with the neighboring Si atoms, nitrogen has one electron that is only loosely bound to the lattice. An electron may be more easily excited into the conduction band (the dopant provides a smaller band gap). With enough dopants (often 1 doping atom per 1 thousand to 1 billion Si atoms) and energy to excite electrons into the conduction band, the material becomes less like an insulator and more like a conductor. -
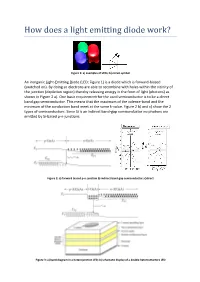
An Inorganic Light-Emitting Diode (LED; Figure 1) Is a Diode Which Is Forward-Biased (Switched On)
Figure 1: a) examples of LEDs; b) circuit symbol An inorganic Light-Emitting Diode (LED; Figure 1) is a diode which is forward-biased (switched on). By doing so electrons are able to recombine with holes within the vicinity of the junction (depletion region) thereby releasing energy in the form of light (photons) as shown in Figure 2 a). One basic requirement for the used semiconductor is to be a direct band gap semiconductor. This means that the maximum of the valence-band and the minimum of the conduction band meet at the same k-value. Figure 2 b) and c) show the 2 types of semiconductors. Since Si is an indirect band-gap semiconductor no photons are emitted by Si-based p-n-junctions. Figure 2: a) forward biased p-n-junction b) indirect band-gap semiconductor; c)direct Figure 3: a) band diagram in a heterojunction LED; b) schematic display of a double heterostructure LED Figure 3 a) shows the band diagram of an improved LED where a heterojuncion is used. The figure shows that the central material (where the light is generated) is surrounded by a region with a higher energy gap. This allows confining the injected charge carriers inside the un-doped central region with a lower band-gap thereby increasing the emissive radiation rate. Figure 3 b) shows the configuration of a double heterorstructure LED. This type of confinement is also utilized for semiconductor lasers. The usage of a heterojunction is further advantageous to obtain a higher output of light out of the LED since the generated photons don’t have enough energy to generate an electron-hole pair within the semiconductor with wider band-gap. -

Molecfit: a General Tool for Telluric Absorption Correction I
A&A 576, A77 (2015) Astronomy DOI: 10.1051/0004-6361/201423932 & c ESO 2015 Astrophysics Molecfit: A general tool for telluric absorption correction I. Method and application to ESO instruments?;?? A. Smette1, H. Sana1;2, S. Noll3, H. Horst1;4, W. Kausch3;5, S. Kimeswenger6;3, M. Barden7, C. Szyszka3, A. M. Jones3, A. Gallenne8, J. Vinther9, P. Ballester9, and J. Taylor9 1 European Southern Observatory, Casilla 19001, Alonso de Cordova 3107 Vitacura, Santiago, Chile e-mail: [email protected] 2 now at ESA/Space Telescope Science Institute, 3700 San Martin Dr, Baltimore, MD 21218, USA 3 Institute for Astro and Particle Physics, Universität Innsbruck, Technikerstrasse 25, 6020 Innsbruck, Austria 4 Josef-Führer-Straße 33, 80997 München, Germany 5 University of Vienna, Department of Astrophysics, Türkenschanzstr. 17 (Sternwarte), 1180 Vienna, Austria 6 Instituto de Astronomía, Universidad Católica del Norte, Avenida Angamos 0610, Antofagasta, Chile 7 International Graduate School of Science and Engineering, Technische Universität München, Boltzmannstr. 17, 85748 Garching bei München, Germany 8 Universidad de Concepción, Casilla 160-C, Concepción, Chile 9 European Southern Observatory, Karl-Schwarzschild-Strasse 2, 85748 Garching bei München, Germany Received 2 April 2014 / Accepted 23 December 2014 ABSTRACT Context. The interaction of the light from astronomical objects with the constituents of the Earth’s atmosphere leads to the formation of telluric absorption lines in ground-based collected spectra. Correcting for these lines, mostly affecting the red and infrared region of the spectrum, usually relies on observations of specific stars obtained close in time and airmass to the science targets, therefore using precious observing time.