The Relationship Between Chewcal Structure and Physiological Response
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
![Downloaded from the Gnomad Browser [36] and Analysed (Supplementary Table S1)](https://docslib.b-cdn.net/cover/6572/downloaded-from-the-gnomad-browser-36-and-analysed-supplementary-table-s1-1896572.webp)
Downloaded from the Gnomad Browser [36] and Analysed (Supplementary Table S1)
International Journal of Molecular Sciences Article Functional Characterisation of Three Glycine N-Acyltransferase Variants and the Effect on Glycine Conjugation to Benzoyl–CoA Johann M. Rohwer 1 , Chantelle Schutte 2 and Rencia van der Sluis 2,* 1 Laboratory for Molecular Systems Biology, Department of Biochemistry, Stellenbosch University, Private Bag X1, Matieland, Stellenbosch 7602, South Africa; [email protected] 2 Focus Area for Human Metabolomics, North-West University, Private Bag X6001, Potchefstroom 2520, South Africa; [email protected] * Correspondence: [email protected]; Tel.: +27-18-299-2068 Abstract: The glycine conjugation pathway in humans is involved in the metabolism of natural sub- strates and the detoxification of xenobiotics. The interactions between the various substrates in this pathway and their competition for the pathway enzymes are currently unknown. The pathway consists of a mitochondrial xenobiotic/medium-chain fatty acid: coenzyme A (CoA) ligase (ACSM2B) and glycine N-acyltransferase (GLYAT). The catalytic mechanism and substrate specificity of both of these enzymes have not been thoroughly characterised. In this study, the level of evolutionary conservation of GLYAT missense variants and haplotypes were analysed. From these data, haplotype variants were selected (156Asn > Ser, [17Ser > Thr,156Asn > Ser] and [156Asn > Ser,199Arg > Cys]) in order to characterise the kinetic mechanism of the enzyme over a wide range of substrate concentra- tions. The 156Asn > Ser haplotype has the highest frequency and the highest relative enzyme activity in all populations studied, and hence was used as the reference in this study. Cooperative substrate Citation: Rohwer, J.M.; Schutte, C.; binding was observed, and the kinetic data were fitted to a two-substrate Hill equation. -

Biochemical Investigations in the Rare Disease Alkaptonuria: Studies on the Metabolome and the Nature of Ochronotic Pigment
Biochemical Investigations in the Rare Disease Alkaptonuria: Studies on the Metabolome and the Nature of Ochronotic Pigment Thesis submitted in accordance with the requirements of the University of Liverpool for the degree of Doctor of Philosophy by Brendan Paul Norman September 2019 ACKNOWLEDGEMENTS It is hard to describe the journey this PhD has taken me on without reverting to well-worn clichés. There has been plenty of challenges along the way, but ultimately I can look back on the past four years with a great sense of pride, both in the work presented here and the skills I have developed. Equally important though are the relationships I have established. I have lots of people to thank for playing a part in this thesis. First, I would like to thank my supervisors, Jim Gallagher, Lakshminarayan Ranganath and Norman Roberts for giving me this fantastic opportunity. Your dedication to research into alkaptonuria (AKU) is inspiring and our discussions together have always been thoughtful and often offered fresh perspective on my work. It has been a pleasure to work under your supervision and your ongoing support and encouragement continues to drive me on. It has truly been a pleasure to be part of the AKU research group. Andrew Davison deserves a special mention - much of the highs and lows of our PhD projects have been experienced together. Learning LC-QTOF-MS was exciting (and continues to be) but equally daunting at the start of our projects (admittedly more so for me as a Psychology graduate turned mass spectrometrist!). I am very proud of what we have achieved together, largely starting from scratch on the instrument, and we are continuing to learn all the time. -

Glycine Conjugates
Industrial Health, 1977, 15, 67. COLORIMETRIC DETERMINATION OF URINARY GLYCINE CONJUGATES Masana OGATA, Reiko SUGIHARA, Hiroko ASAHARA and Shohei KIRA Department of Public Health, Okayama University Medical School, Shikatacho, Okayama 700 Japan (Received January 26, 1977) A quantitative procedure is described for determining urinary glycine conjugates. The color development was recognized after addition of pyridine and benzenesul- fonyl chloride to glycine conjugates. The dried residue of ethyl acetate extract from urine was dissolved in pyridine and benzenesulfonyl chloride was added. After addition of chloroform to the mixture, the absorbance was determined at 420 nm (extracting method). In the improved procedure of direct colorimetric method, pyridine was added to urine and mixed; then benzenesulfonyl chloride was diluted with mixture of dimethylsulfoxide and ethanol, and absorbance was deter- mined at 430 nm (improved direct method). To separate each glycine conjugate, paper chromatographic procedure was car- ried out. Urine specimen each was directly developed on filter paper with butanol•\ acetic acid•\water or isopropanol•\ammonia•\water. On the other hand, ethyl acetate extract from urine was developed with toluene•\cetic acid•\water. Comparing the spots with authentic sample spots, each location was determined by illuminating with ultraviolet light. Then, each spot was extracted with pyridine. Benzen- sulfonyl chloride was added to the extract; the absorbance was determined at 420 nm. Complete separation of urinary glycine conjugates -

Study of the Paradoxical Effects of Salicylate in Low, Intermediate and High Dosage on the Renal Mechanisms for Excretion of Urate in Man
STUDY OF THE PARADOXICAL EFFECTS OF SALICYLATE IN LOW, INTERMEDIATE AND HIGH DOSAGE ON THE RENAL MECHANISMS FOR EXCRETION OF URATE IN MAN T'Sai Fan Yü, Alexander B. Gutman J Clin Invest. 1959;38(8):1298-1315. https://doi.org/10.1172/JCI103905. Research Article Find the latest version: https://jci.me/103905/pdf STUDY OF THE PARADOXICAL EFFECTS OF SALICYLATE IN LOW, INTERMEDIATE AND HIGH DOSAGE ON THE RENAL MECHANISMS FOR EXCRETION OF URATE IN MAN * By T'SAI FAN YU AND ALEXANDER B. GUTMAN (From the Departments of Medicine, The Mount Sinai Hospital, and Columbia University College o. Physicians and Surgeons, New York, N. Y.) (Submitted for publication February 2, 1959; accepted April 2, 1959) Salicylate administered to man in sufficiently of a salicylate metabolite, gentisic acid. The re- large dosage (5 to 6 or more Gm. per day) causes lationships between the renal excretion of salicyl- marked uricosuria, characterized by substan- ate and of urate are then examined by means of tially increased urate/inulin clearance ratios at- simultaneous clearance techniques, including tributable to inhibition of tubular reabsorption of studies under conditions of urine pH made to the filtered urate. In smaller dosage (1 to 2 Gm. vary from markedly acid to markedly alkaline. per day) salicylate exerts a contrary effect, re- Clearance data on the antagonistic effect of small tention of urate, associated with lower than nor- doses of salicylate on probenecid uricosuria, and mal urate/inulin clearance ratios (1-8). When vice versa, also are presented. Finally, the im- small doses of salicylate are given concurrently plications of the results as a whole are considered with probenecid, the retention of urate caused by in relation to current concepts of renal mecha- the salicylate is sufficiently pronounced to nisms for the excretion of salicylate and of urate, counteract, to a marked degree, the uricosuric with special reference to inferences regarding the effect of the probenecid (9, 10). -

Toxicological Profile for Xylene
TOXICOLOGICAL PROFILE FOR XYLENE U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service Agency for Toxic Substances and Disease Registry August 2007 XYLENE ii DISCLAIMER The use of company or product name(s) is for identification only and does not imply endorsement by the Agency for Toxic Substances and Disease Registry. XYLENE iii UPDATE STATEMENT A Toxicological Profile for Xylene, Draft for Public Comment was released in September 2005. This edition supersedes any previously released draft or final profile. Toxicological profiles are revised and republished as necessary. For information regarding the update status of previously released profiles, contact ATSDR at: Agency for Toxic Substances and Disease Registry Division of Toxicology and Environmental Medicine/Applied Toxicology Branch 1600 Clifton Road NE Mailstop F-32 Atlanta, Georgia 30333 XYLENE iv This page is intentionally blank. v FOREWORD This toxicological profile is prepared in accordance with guidelines developed by the Agency for Toxic Substances and Disease Registry (ATSDR) and the Environmental Protection Agency (EPA). The original guidelines were published in the Federal Register on April 17, 1987. Each profile will be revised and republished as necessary. The ATSDR toxicological profile succinctly characterizes the toxicologic and adverse health effects information for the hazardous substance described therein. Each peer-reviewed profile identifies and reviews the key literature that describes a hazardous substance's toxicologic properties. Other pertinent literature is also presented, but is described in less detail than the key studies. The profile is not intended to be an exhaustive document; however, more comprehensive sources of specialty information are referenced. The focus of the profiles is on health and toxicologic information; therefore, each toxicological profile begins with a public health statement that describes, in nontechnical language, a substance's relevant toxicological properties. -

408A Rubber Renue
408A Rubber Renue MG Chemicals UK Limited Version No: A-1.0 2 Issue Date:27/02/2018 Safety Data Sheet (Conforms to Regulation (EU) No 2015/830) Revision Date: 14/09/2020 L.REACH.GBR.EN SECTION 1 IDENTIFICATION OF THE SUBSTANCE / MIXTURE AND OF THE COMPANY / UNDERTAKING 1.1. Product Identifier Product name 408A Rubber Renue Synonyms SDS Code: 408A-Liquid; 408A-100ML, 408A-125ML, 408A-250ML, 408A-1L | UFI: V890-J061-A00J-U0DD Other means of identification Not Available 1.2. Relevant identified uses of the substance or mixture and uses advised against Relevant identified uses Liquid for rejuvenating and reconditioning rubber Uses advised against Not Applicable 1.3. Details of the supplier of the safety data sheet Registered company name MG Chemicals UK Limited MG Chemicals (Head office) Heame House, 23 Bilston Street, Sedgely Dudley DY3 1JA United Address 9347 - 193 Street Surrey V4N 4E7 British Columbia Canada Kingdom Telephone +(44) 1663 362888 +(1) 800-201-8822 Fax Not Available +(1) 800-708-9888 Website Not Available www.mgchemicals.com Email [email protected] [email protected] 1.4. Emergency telephone number Association / Organisation Verisk 3E (Access code: 335388) Not Available Emergency telephone numbers +(44) 20 35147487 Not Available Other emergency telephone +(0) 800 680 0425 Not Available numbers SECTION 2 HAZARDS IDENTIFICATION 2.1. Classification of the substance or mixture H226 - Flammable Liquid Category 3, H315 - Skin Corrosion/Irritation Category 2, H319 - Eye Irritation Category 2, H351 - Carcinogenicity Category 2, Classification according to H335 - Specific target organ toxicity - single exposure Category 3 (respiratory tract irritation), H336 - Specific target organ toxicity - single exposure regulation (EC) No 1272/2008 Category 3 (narcotic effects), H373 - Specific target organ toxicity - repeated exposure Category 2, H304 - Aspiration Hazard Category 1, H412 - Chronic [CLP] [1] Aquatic Hazard Category 3 1. -
1 in Silico Prediction of Skin Metabolism and Its
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by LJMU Research Online In Silico Prediction of Skin Metabolism and its Implication in Toxicity Assessment JC Maddena, S Webbb, SJ Enocha, HE Colleyc, C Murdochc, R Shipleyd, P Sharmae, C Yangf, and MTD Cronina aSchool of Pharmacy and Biomolecular Sciences, Liverpool John Moores University, Byrom Street, Liverpool, L3 3AF, UK; bDepartment of Applied Mathematics, Liverpool John Moores University, Byrom Street, Liverpool L3 3AF, UK; cSchool of Clinical Dentistry, University of Sheffield, Claremont Crescent, Sheffield, S10 2TA, UK, dDepartment of Mechanical Engineering, University College London, Gower Street, London, WC1E 6BT, UK; eDepartment of Molecular and Clinical Pharmacology, MRC Centre for Drug Safety Science, Institute of Translational Medicine, University of Liverpool, Sherrington Building, Liverpool, L69 3GE, UK; fMolecular Networks GmbH - Computerchemie, Henkestrasse 91, 91052 Erlangen, Germany Corresponding author: Judith Madden E-mail: [email protected] Tel: +44 (0)151 231 2032 Abstract Skin, being the largest organ of the body, represents an important route of exposure, not only for the abundance of chemicals present in the environment, but also for products designed for topical application such as drugs and personal care products. Determining whether such incidental or intentional exposure poses a risk to human health requires consideration of temporal concentration, both externally and internally, in addition to assessing the chemical’s intrinsic hazard. In order to elicit a toxic response in vivo the chemical must reach its site of action in sufficient concentration, as determined by its absorption, distribution, metabolism and elimination (ADME) profile. -
Salicylate in Man by Carole Bedford, A
Brit. J. Pharmacol. (1965), 24, 418-431. A KINETIC STUDY OF THE ELIMINATION OF SALICYLATE IN MAN BY CAROLE BEDFORD, A. J. CUMMINGS AND B. K. MARTIN From the Nicholas Research Institute, Slough, Bucks. (Received July 20, 1964) The elimination of salicylate in man is a slow process, after a single 1 g dose of aspirin the average half-life of salicylate is reported to be 6 hr (Brodie, Burns & Weiner, 1959), while after very high doses a value of 19 hr has been obtained by Swintosky (1956). It can be inferred that the half-life of salicylate varies with dosage, that is with body salicylate concentration, and therefore that elimination cannot be invariably described by first-order kinetics and the ratio in which the metabolites are formed may change with dosage and time after dosage. Only a small proportion of the dose is normally excreted directly as unconjugated salicy- late and the removal of salicylate from the body is almost entirely dependent upon the formation of the metabolites salicyluric acid and the two glucuronides of salicylic acid. Salicyluric acid is the major metabolite and may account for more than half the total metabolites of salicylic acid excreted in the urine (Baldoni, 1915; Smith, Gleason, Stoll & Ogorzalek, 1946; Salassa, Bollman & Dry, 1948; Schachter & Manis, 1958). It has been previously suggested that the rate of formation of salicyluric acid is limited when the plasma salicylate concentration exceeds some low level (Cummings, 1963). The present paper describes a study of the rate of " total salicylate " and salicyluric acid excretion in the urine after various doses of aspirin. -
Interactions Ofm-Xylene and Aspirin Metabolism In
Br J Ind Med: first published as 10.1136/oem.45.2.127 on 1 February 1988. Downloaded from British Journal ofIndustrial Medicine 1988;45:127-132 Interactions of m-xylene and aspirin metabolism in man LYNN CAMPBELL,' H KERR WILSON,' A MARGARET SAMUEL,2 D GOMPERTZ' From the Occupational Medicine and Hygiene Laboratories,' London NW2 6LN, and Employment Medical Advisory Centre, 2 Essex IGII 8HF, UK ABSTRACT In a series of experiments to investigate interactions between industrial solvents and common medications the interaction between m-xylene and aspirin was studied. As both these substances are metabolised and excreted as glycine conjugates there would possibly be competition for this conjugation pathway. Five male volunteers were exposed on separate occasions to m-xylene by inhalation (100 ppm), aspirin (1500 mg) by mouth, and m-xylene and aspirin together under controlled conditions in an exposure chamber. Urine and blood samples were collected and analysed for m-xylene, aspirin, and their metabolites. The amounts of the major glycine conjugates produced from m-xylene (m-methylhippuric acid) and aspirin (salicyluric acid) were significantly reduced by about 50% when m-xylene and aspirin were coadministered. There appears to be a mutual inhibition on the formation of the respective glycine conjugates. It is suggested that the inhibition is due to competition for either the enzymes, acyl-CoA synthetase, or glycine N-acylase. These findings have implications in the biological monitoring of workers exposed to m-xylene. copyright. The interaction between solvents and ethanol is well that may lead to taking analgesics containing aspirin known'3 but there have been few reports on the (acetylsalicylic acid). -

Clh Rep Methyl Salicylate En.Pdf
CLH REPORT FOR [METHYL SALICYLATE] CLH report Proposal for Harmonised Classification and Labelling Based on Regulation (EC) No 1272/2008 (CLP Regulation), Annex VI, Part 2 International Chemical Identification: Methyl salicylate EC Number: 204-317-7 CAS Number: 119-36-8 Index Number: - Contact details for dossier submitter: ANSES (on behalf of the French MSCA) 14 rue Pierre Marie Curie F-94701 Maisons-Alfort Cedex [email protected] Version number: v1 Date: June 2018 CLH REPORT FOR [METHYL SALICYLATE] CONTENTS 1 IDENTITY OF THE SUBSTANCE ...................................................................................................................... 1 1.1 NAME AND OTHER IDENTIFIERS OF THE SUBSTANCE .............................................................................................. 1 1.2 COMPOSITION OF THE SUBSTANCE ........................................................................................................................ 2 2 PROPOSED HARMONISED CLASSIFICATION AND LABELLING ........................................................... 3 2.1 PROPOSED HARMONISED CLASSIFICATION AND LABELLING ACCORDING TO THE CLP CRITERIA ........................... 3 3 HISTORY OF THE PREVIOUS CLASSIFICATION AND LABELLING ..................................................... 5 4 JUSTIFICATION THAT ACTION IS NEEDED AT COMMUNITY LEVEL ................................................ 5 5 IDENTIFIED USES .............................................................................................................................................. -
CPY Document
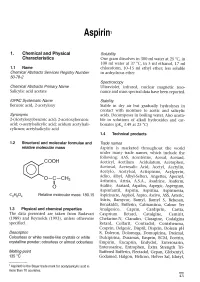
Aspirin1 1. Chemical and Physical Solubility Characteristics One gram dissolves in 300 ml water at 25°C, in 100 ml water at 37°C, in 5 ml ethanol, 17 ml 1.1 Narne chloroform, 10-15 ml ethyl ether; less soluble Chemical Abstracts Services Registry Number in anhydrous ether 50-78-2 Spectroscopy Chemical Abstracts Primary Name Ultraviolet, infrared, nuclear magnetic reso- Salicylic acid acetate nance and mass spectral data have been reported. IUPAC Systematic Name Stabilty Benzoic acid, 2-acetyloxy Stable in dry air but gradually hydrolyses in contact with moisture to acetic and salicylic Synonyms acids. Decomposes in boilng water. Also unsta- 2-(Acetyloxy)benzoic acid; 2-acetoxybenzoic ble in solutions of alkali hydroxides and car- acid; o-acetylsalicylic acid; acidum acetylsali- bonates (pKa 3.49 at 25°C) cylicum; acetylsalicylic acid 1.4 Technical products 1.2 Structural and rnolecular forrnulae and Trade names relative rnolecular rnass Aspirin is marketed throughout the world under many trade names, which include the foIlowing: AAS, Acentérine, Acesal, Acetard, COOH Aceticyl, Acetilum Acidulatum, Acetophen, Acetosal, Acetosalic Acid, Acetyl, Acetylin, Acetylo, Acetylsal, Actispirine, Acylpyrin, Adiro, Albyl, Albyl-Selters, Angettes, Apernyl, ~O-C-CH3 Arthrisin, Artria, A.S.A., Asadrine, Asaferm, oIl Asalite, Asatard, Aspalox, Aspegic, Aspergum, Aspinfantil, Aspirin, Aspirina, Aspirinetta, CgHa04 Relative molecular mass: 180.15 Aspirisucre, Aspisol, Aspro, Asrivo, ASS, Asteric, Astrix, Bamycor, Bamyl, Bamyl S, Bebesan, Bonakiddi, Bufferin, Calmantina, Calmo Yer 1.3 Physical and chernical properties Analgesico, Caprin, Cardiprin, Cartia, The data presented are taken from Budavari Casprium Retard, Catalgine, Cemirit, (1989) and Reynolds (1993), unless otherwise Chefarine-N, Claradin, Claragine, Codalgina specified. -

Interactions Ofm-Xylene and Aspirin Metabolism In
Br J Ind Med: first published as 10.1136/oem.45.2.127 on 1 February 1988. Downloaded from British Journal ofIndustrial Medicine 1988;45:127-132 Interactions of m-xylene and aspirin metabolism in man LYNN CAMPBELL,' H KERR WILSON,' A MARGARET SAMUEL,2 D GOMPERTZ' From the Occupational Medicine and Hygiene Laboratories,' London NW2 6LN, and Employment Medical Advisory Centre, 2 Essex IGII 8HF, UK ABSTRACT In a series of experiments to investigate interactions between industrial solvents and common medications the interaction between m-xylene and aspirin was studied. As both these substances are metabolised and excreted as glycine conjugates there would possibly be competition for this conjugation pathway. Five male volunteers were exposed on separate occasions to m-xylene by inhalation (100 ppm), aspirin (1500 mg) by mouth, and m-xylene and aspirin together under controlled conditions in an exposure chamber. Urine and blood samples were collected and analysed for m-xylene, aspirin, and their metabolites. The amounts of the major glycine conjugates produced from m-xylene (m-methylhippuric acid) and aspirin (salicyluric acid) were significantly reduced by about 50% when m-xylene and aspirin were coadministered. There appears to be a mutual inhibition on the formation of the respective glycine conjugates. It is suggested that the inhibition is due to competition for either the enzymes, acyl-CoA synthetase, or glycine N-acylase. These findings have implications in the biological monitoring of workers exposed to m-xylene. copyright. The interaction between solvents and ethanol is well that may lead to taking analgesics containing aspirin known'3 but there have been few reports on the (acetylsalicylic acid).