GWAS-Driven Pathway Analyses and Functional Validation Suggest GLIS1 As
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Deregulated Gene Expression Pathways in Myelodysplastic Syndrome Hematopoietic Stem Cells
Leukemia (2010) 24, 756–764 & 2010 Macmillan Publishers Limited All rights reserved 0887-6924/10 $32.00 www.nature.com/leu ORIGINAL ARTICLE Deregulated gene expression pathways in myelodysplastic syndrome hematopoietic stem cells A Pellagatti1, M Cazzola2, A Giagounidis3, J Perry1, L Malcovati2, MG Della Porta2,MJa¨dersten4, S Killick5, A Verma6, CJ Norbury7, E Hellstro¨m-Lindberg4, JS Wainscoat1 and J Boultwood1 1LRF Molecular Haematology Unit, NDCLS, John Radcliffe Hospital, Oxford, UK; 2Department of Hematology Oncology, University of Pavia Medical School, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy; 3Medizinische Klinik II, St Johannes Hospital, Duisburg, Germany; 4Division of Hematology, Department of Medicine, Karolinska Institutet, Stockholm, Sweden; 5Department of Haematology, Royal Bournemouth Hospital, Bournemouth, UK; 6Albert Einstein College of Medicine, Bronx, NY, USA and 7Sir William Dunn School of Pathology, University of Oxford, Oxford, UK To gain insight into the molecular pathogenesis of the the World Health Organization.6,7 Patients with refractory myelodysplastic syndromes (MDS), we performed global gene anemia (RA) with or without ringed sideroblasts, according to expression profiling and pathway analysis on the hemato- poietic stem cells (HSC) of 183 MDS patients as compared with the the French–American–British classification, were subdivided HSC of 17 healthy controls. The most significantly deregulated based on the presence or absence of multilineage dysplasia. In pathways in MDS include interferon signaling, thrombopoietin addition, patients with RA with excess blasts (RAEB) were signaling and the Wnt pathways. Among the most signifi- subdivided into two categories, RAEB1 and RAEB2, based on the cantly deregulated gene pathways in early MDS are immuno- percentage of bone marrow blasts. -

PARSANA-DISSERTATION-2020.Pdf
DECIPHERING TRANSCRIPTIONAL PATTERNS OF GENE REGULATION: A COMPUTATIONAL APPROACH by Princy Parsana A dissertation submitted to The Johns Hopkins University in conformity with the requirements for the degree of Doctor of Philosophy Baltimore, Maryland July, 2020 © 2020 Princy Parsana All rights reserved Abstract With rapid advancements in sequencing technology, we now have the ability to sequence the entire human genome, and to quantify expression of tens of thousands of genes from hundreds of individuals. This provides an extraordinary opportunity to learn phenotype relevant genomic patterns that can improve our understanding of molecular and cellular processes underlying a trait. The high dimensional nature of genomic data presents a range of computational and statistical challenges. This dissertation presents a compilation of projects that were driven by the motivation to efficiently capture gene regulatory patterns in the human transcriptome, while addressing statistical and computational challenges that accompany this data. We attempt to address two major difficulties in this domain: a) artifacts and noise in transcriptomic data, andb) limited statistical power. First, we present our work on investigating the effect of artifactual variation in gene expression data and its impact on trans-eQTL discovery. Here we performed an in-depth analysis of diverse pre-recorded covariates and latent confounders to understand their contribution to heterogeneity in gene expression measurements. Next, we discovered 673 trans-eQTLs across 16 human tissues using v6 data from the Genotype Tissue Expression (GTEx) project. Finally, we characterized two trait-associated trans-eQTLs; one in Skeletal Muscle and another in Thyroid. Second, we present a principal component based residualization method to correct gene expression measurements prior to reconstruction of co-expression networks. -

Transcriptomic Analysis of Pluripotent Stem Cells: Insights Into Health and Disease Jia-Chi Yeo1,2 and Huck-Hui Ng1,2,3,4,5,*
Yeo and Ng Genome Medicine 2011, 3:68 http://genomemedicine.com/content/3/10/68 REVIEW Transcriptomic analysis of pluripotent stem cells: insights into health and disease Jia-Chi Yeo1,2 and Huck-Hui Ng1,2,3,4,5,* Abstract types, termed ‘pluripotency’, allows researchers to study early mammalian development in an artificial setting and Embryonic stem cells (ESCs) and induced pluripotent offers opportunities for regenerative medicine, whereby stem cells (iPSCs) hold tremendous clinical potential ESCs could generate clinically relevant cell types for because of their ability to self-renew, and to tissue repair. However, this same malleability of ESCs dierentiate into all cell types of the body. This unique also renders it a challenge to obtain in vitro differentiation capacity of ESCs and iPSCs to form all cell lineages of ESCs to specific cell types at high efficacy. erefore, is termed pluripotency. While ESCs and iPSCs are harnessing the full potential of ESCs requires an in-depth pluripotent and remarkably similar in appearance, understanding of the factors and mechanisms regulating whether iPSCs truly resemble ESCs at the molecular ESC pluripotency and cell lineage decisions. level is still being debated. Further research is therefore Early studies on ESCs led to the discovery of the core needed to resolve this issue before iPSCs may be safely pluripotency factors Oct4, Sox2 and Nanog [1], and, applied in humans for cell therapy or regenerative increasingly, the use of genome-level screening assays has medicine. Nevertheless, the use of iPSCs as an in vitro revealed new insights by uncovering additional trans- human genetic disease model has been useful in cription factors, transcriptional cofactors and chromatin studying the molecular pathology of complex genetic remodeling complexes involved in the maintenance of diseases, as well as facilitating genetic or drug screens. -
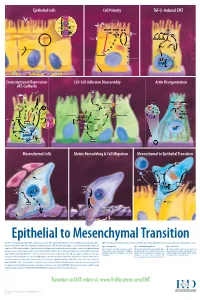
Epithelial to Mesenchymal Transition
Epithelial Cells Cell Polarity TGF-b-Induced EMT MUC-1 O-glycosylation Epithelial Cells ZO-1 Occludin Apical Membrane Tight F-Actin Microvilli Junction Claudin F-Actin p120 β-Catenin Adherens F-Actin Ezrin TGF-β dimer Junction E-Cadherin α-Catenin Plakophilin Crumbs Complex PAR Complex Desmocollin Desmoplakin Desmosome PtdIns(4,5)P2 TGF-β RII TGF-β RI CRB Cdc42Par6 Desmoglein Cytokeratin Pals1 PatJ Tight Junction Plakoglobin aPKC Par3 Domain Smad7 Extracellular PTEN JNK ERK1/2 p38 SARA Smurf1 Cortical Actin Cytoskeleton Space Par3 ZO-1 Adherens Junction PI 3-K Domain Smad-independent Signaling (–) Smad7 Translocation Smad2/3 PtdIns(3,4,5)P3 Smad4 Smad4 NEDD4 Cytokeratin Intermediate Filaments Smad2 Smad4 Smad3 LLGL Proteasome SCRIB DLG Scribble Complex Fibronectin Twist Smad2/3 Vitronectin ZEB 1/2 Microtubule Network Smad4 N-Cadherin Snail Basolateral Membrane CoA, Collagen I Slug CoR MMPs DNA-binding (+) Claudin Desmoplakin Transcription Factor Occludin Cytokeratins E-Cadherin Plakoglobin Integrins β α Nidogen-1/Entactin Perlecan Laminin Collagen IV Transcriptional Repression Cell-Cell Adhesion Disassembly Actin Reorganization of E-Cadherin TGF-β dimer EGF TGF-β RII TGF-β RI IGF FGF Receptor TNF-α Tyrosine Kinase Par6 TNF RI Apical Focal Adhesion Constriction Actin Depolymerization F-Actin Smurf1 Occludin Wnt Frizzled Myosin II Ras RhoA α-Actinin Myosin II ROCK AxinCK1 Dishevelled GSK-3 PI 3-K Src Zyxin MLC Phosphatase APC Proteasome FAK Vinculin RhoA ILK Talin (Inactive) Hakai Talin FAK F-Actin E-Cadherin LIMK Akt Paxillin FAK Stress -

MUC4/MUC16/Muc20high Signature As a Marker of Poor Prognostic for Pancreatic, Colon and Stomach Cancers
Jonckheere and Van Seuningen J Transl Med (2018) 16:259 https://doi.org/10.1186/s12967-018-1632-2 Journal of Translational Medicine RESEARCH Open Access Integrative analysis of the cancer genome atlas and cancer cell lines encyclopedia large‑scale genomic databases: MUC4/MUC16/ MUC20 signature is associated with poor survival in human carcinomas Nicolas Jonckheere* and Isabelle Van Seuningen* Abstract Background: MUC4 is a membrane-bound mucin that promotes carcinogenetic progression and is often proposed as a promising biomarker for various carcinomas. In this manuscript, we analyzed large scale genomic datasets in order to evaluate MUC4 expression, identify genes that are correlated with MUC4 and propose new signatures as a prognostic marker of epithelial cancers. Methods: Using cBioportal or SurvExpress tools, we studied MUC4 expression in large-scale genomic public datasets of human cancer (the cancer genome atlas, TCGA) and cancer cell line encyclopedia (CCLE). Results: We identifed 187 co-expressed genes for which the expression is correlated with MUC4 expression. Gene ontology analysis showed they are notably involved in cell adhesion, cell–cell junctions, glycosylation and cell signal- ing. In addition, we showed that MUC4 expression is correlated with MUC16 and MUC20, two other membrane-bound mucins. We showed that MUC4 expression is associated with a poorer overall survival in TCGA cancers with diferent localizations including pancreatic cancer, bladder cancer, colon cancer, lung adenocarcinoma, lung squamous adeno- carcinoma, skin cancer and stomach cancer. We showed that the combination of MUC4, MUC16 and MUC20 signature is associated with statistically signifcant reduced overall survival and increased hazard ratio in pancreatic, colon and stomach cancer. -

A Computational Study of Stress Fiber-Focal Adhesion Dynamics
A computational study of stress fiber-focal adhesion dynamics governing cell contractility M. Maraldi1, C. Valero2, K. Garikipati1;3∗ 1Department of Mechanical Engineering, University of Michigan, Ann Arbor, Michigan 2M2BE, Aragon´ Institute of Engineering Research (I3A), University of Zaragoza, Zaragoza, Spain 3Department of Mathematics, University of Michigan, Ann Arbor, Michigan Abstract We apply a recently developed model of cytoskeletal force generation to study a cell’s intrinsic contractility, as well as its response to external loading. The model is based on a non-equilibrium thermodynamic treatment of the mechano-chemistry governing force in the stress fiber-focal adhesion system. Our computational study suggests that the mechan- ical coupling between the stress fibers and focal adhesions leads to a complex, dynamic, mechano-chemical response. We collect the results in response maps whose regimes are distinguished by the initial geometry of the stress fiber-focal adhesion system, and by the external load on the cell. The results from our model connect qualitatively with recent stud- ies on the force response of smooth muscle cells on arrays of polymeric microposts (Mann et al., Lab. on a Chip, 12, 731-740, 2012). INTRODUCTION In contractile cells, such as smooth muscle cells and fibroblasts, the generation of traction force is the result of two different actions: myosin-powered cytoskeletal contractility and external mechanical stimuli (applied stretch or force). The cooperation between these two aspects de- termines the level of the force within the cell and influences the development of cytoskeletal components via the (un)binding of proteins. Important cytoskeletal components that mediate this interplay of mechanics and chemistry are stress fibers and focal adhesions. -

Aneuploidy: Using Genetic Instability to Preserve a Haploid Genome?
Health Science Campus FINAL APPROVAL OF DISSERTATION Doctor of Philosophy in Biomedical Science (Cancer Biology) Aneuploidy: Using genetic instability to preserve a haploid genome? Submitted by: Ramona Ramdath In partial fulfillment of the requirements for the degree of Doctor of Philosophy in Biomedical Science Examination Committee Signature/Date Major Advisor: David Allison, M.D., Ph.D. Academic James Trempe, Ph.D. Advisory Committee: David Giovanucci, Ph.D. Randall Ruch, Ph.D. Ronald Mellgren, Ph.D. Senior Associate Dean College of Graduate Studies Michael S. Bisesi, Ph.D. Date of Defense: April 10, 2009 Aneuploidy: Using genetic instability to preserve a haploid genome? Ramona Ramdath University of Toledo, Health Science Campus 2009 Dedication I dedicate this dissertation to my grandfather who died of lung cancer two years ago, but who always instilled in us the value and importance of education. And to my mom and sister, both of whom have been pillars of support and stimulating conversations. To my sister, Rehanna, especially- I hope this inspires you to achieve all that you want to in life, academically and otherwise. ii Acknowledgements As we go through these academic journeys, there are so many along the way that make an impact not only on our work, but on our lives as well, and I would like to say a heartfelt thank you to all of those people: My Committee members- Dr. James Trempe, Dr. David Giovanucchi, Dr. Ronald Mellgren and Dr. Randall Ruch for their guidance, suggestions, support and confidence in me. My major advisor- Dr. David Allison, for his constructive criticism and positive reinforcement. -

Endothelial Cadherin Endothelium Is Regulated by Vascular Progenitor
Migration of Human Hematopoietic Progenitor Cells Across Bone Marrow Endothelium Is Regulated by Vascular Endothelial Cadherin This information is current as of October 1, 2021. Jaap D. van Buul, Carlijn Voermans, Veronique van den Berg, Eloise C. Anthony, Frederik P. J. Mul, Sandra van Wetering, C. Ellen van der Schoot and Peter L. Hordijk J Immunol 2002; 168:588-596; ; doi: 10.4049/jimmunol.168.2.588 http://www.jimmunol.org/content/168/2/588 Downloaded from References This article cites 54 articles, 33 of which you can access for free at: http://www.jimmunol.org/content/168/2/588.full#ref-list-1 http://www.jimmunol.org/ Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication by guest on October 1, 2021 *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2002 by The American Association of Immunologists All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. Migration of Human Hematopoietic Progenitor Cells Across Bone Marrow Endothelium Is Regulated by Vascular Endothelial Cadherin1 Jaap D. van Buul,* Carlijn Voermans,* Veronique van den Berg,* Eloise C. -

Effects of Downregulating GLIS1 Transcript On
Journal of Reproduction and Development, Vol. 61, No 5, 2015 —Original Article— Effects of downregulating GLIS1 transcript on preimplantation development and gene expression of bovine embryos Kazuki TAKAHASHI1), Nobuyuki SAKURAI2), Natsuko EMURA1), Tsutomu HASHIZUME1, 2) and Ken SAWAI1, 2) 1)Faculty of Agriculture, Iwate University, Iwate 020-8550, Japan 2)The United Graduate School of Agricultural Sciences, Iwate University, Iwate 020-8550, Japan Abstract. Krüppel-like protein Gli-similar 1 (GLIS1) is known as a direct reprogramming factor for the generation of induced pluripotent stem cells. The objective of this study was to investigate the role of GLIS1 in the preimplantation development of bovine embryos. GLIS1 transcripts in in vitro-matured oocytes and 1-cell to 4-cell stage embryos were detected, but they were either absent or at trace levels at the 8-cell to blastocyst stages. We attempted GLIS1 downregulation of bovine early embryos by RNA interference and evaluated developmental competency and gene transcripts, which are involved in zygotic gene activation (ZGA) in GLIS1-downregulated embryos. Injection of specific siRNA resulted in a distinct decrease inGLIS1 transcript in bovine embryos at the 4-cell stage. Although the bovine embryos injected with GLIS1-siRNA could develop to the 16-cell stage, these embryos had difficulty in developing beyond the 32-cell stage. Gene transcripts of PDHA1 and HSPA8, which are transcribed after ZGA, showed lower level in GLIS1 downregulated embryos. It is possible that GLIS1- downregulated embryos fail to initiate ZGA. Our results indicated that GLIS1 is an important factor for the preimplantation development of bovine embryos. Key words: Bovine embryo, Early development, Gene expression, GLIS1, RNA interference (J. -

Download Thesis
This electronic thesis or dissertation has been downloaded from the King’s Research Portal at https://kclpure.kcl.ac.uk/portal/ The Genetics and Spread of Amyotrophic Lateral Sclerosis Jones, Ashley Richard Awarding institution: King's College London The copyright of this thesis rests with the author and no quotation from it or information derived from it may be published without proper acknowledgement. END USER LICENCE AGREEMENT Unless another licence is stated on the immediately following page this work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International licence. https://creativecommons.org/licenses/by-nc-nd/4.0/ You are free to copy, distribute and transmit the work Under the following conditions: Attribution: You must attribute the work in the manner specified by the author (but not in any way that suggests that they endorse you or your use of the work). Non Commercial: You may not use this work for commercial purposes. No Derivative Works - You may not alter, transform, or build upon this work. Any of these conditions can be waived if you receive permission from the author. Your fair dealings and other rights are in no way affected by the above. Take down policy If you believe that this document breaches copyright please contact [email protected] providing details, and we will remove access to the work immediately and investigate your claim. Download date: 07. Oct. 2021 THE GENETICS AND SPREAD OF AMYOTROPHIC LATERAL SCLEROSIS Ashley Richard Jones PhD in Clinical Neuroscience - 1 - Abstract Our knowledge of the genetic contribution to Amyotrophic Lateral Sclerosis (ALS) is rapidly growing, and there is increasing research into how ALS spreads through the motor system and beyond. -

Identification of Differentially Expressed Genes in Human Bladder Cancer Through Genome-Wide Gene Expression Profiling
521-531 24/7/06 18:28 Page 521 ONCOLOGY REPORTS 16: 521-531, 2006 521 Identification of differentially expressed genes in human bladder cancer through genome-wide gene expression profiling KAZUMORI KAWAKAMI1,3, HIDEKI ENOKIDA1, TOKUSHI TACHIWADA1, TAKENARI GOTANDA1, KENGO TSUNEYOSHI1, HIROYUKI KUBO1, KENRYU NISHIYAMA1, MASAKI TAKIGUCHI2, MASAYUKI NAKAGAWA1 and NAOHIKO SEKI3 1Department of Urology, Graduate School of Medical and Dental Sciences, Kagoshima University, 8-35-1 Sakuragaoka, Kagoshima 890-8520; Departments of 2Biochemistry and Genetics, and 3Functional Genomics, Graduate School of Medicine, Chiba University, 1-8-1 Inohana, Chuo-ku, Chiba 260-8670, Japan Received February 15, 2006; Accepted April 27, 2006 Abstract. Large-scale gene expression profiling is an effective CKS2 gene not only as a potential biomarker for diagnosing, strategy for understanding the progression of bladder cancer but also for staging human BC. This is the first report (BC). The aim of this study was to identify genes that are demonstrating that CKS2 expression is strongly correlated expressed differently in the course of BC progression and to with the progression of human BC. establish new biomarkers for BC. Specimens from 21 patients with pathologically confirmed superficial (n=10) or Introduction invasive (n=11) BC and 4 normal bladder samples were studied; samples from 14 of the 21 BC samples were subjected Bladder cancer (BC) is among the 5 most common to microarray analysis. The validity of the microarray results malignancies worldwide, and the 2nd most common tumor of was verified by real-time RT-PCR. Of the 136 up-regulated the genitourinary tract and the 2nd most common cause of genes we detected, 21 were present in all 14 BCs examined death in patients with cancer of the urinary tract (1-7). -

Regular Article
From www.bloodjournal.org by guest on April 6, 2015. For personal use only. Regular Article IMMUNOBIOLOGY Hemophagocytic lymphohistiocytosis caused by dominant-negative mutations in STXBP2 that inhibit SNARE-mediated membrane fusion Waldo A. Spessott,1 Maria L. Sanmillan,1 Margaret E. McCormick,1 Nishant Patel,2 Joyce Villanueva,3 Kejian Zhang,4 Kim E. Nichols,5 and Claudio G. Giraudo1 1Department of Pathology and Laboratory Medicine, and 2Division of Oncology, Department of Pediatrics, The Children’s Hospital of Philadelphia, University of Pennsylvania, Philadelphia, PA; 3Division of Bone Marrow Transplant and Immune Deficiency, and 4Division of Human Genetics, Cincinnati Children’s Hospital Medical Center, Department of Pediatrics, University of Cincinnati College of Medicine, Cincinnati, OH; and 5Division of Cancer Predisposition, Department of Oncology, St. Jude Children’s Research Hospital, Memphis, TN Key Points Familial hemophagocytic lymphohistiocytosis (F-HLH) and Griscelli syndrome type 2 (GS) are life-threatening immunodeficiencies characterized by impaired cytotoxic T lymphocyte • Monoallelic STXBP2 mutations (CTL) and natural killer (NK) cell lytic activity. In the majority of cases, these disorders are affecting codon 65 impair caused by biallelic inactivating germline mutations in genes such as RAB27A (GS) and PRF1, lymphocyte cytotoxicity and UNC13D, STX11,andSTXBP2 (F-HLH). Although monoallelic (ie, heterozygous) mutations contribute to hemophagocytic have been identified in certain patients, the clinical significance and molecular mechanisms lymphohistiocytosis. by which these mutations influence CTL and NK cell function remain poorly understood. • Munc18-2R65Q/W mutant Here, we characterize 2 novel monoallelic hemophagocytic lymphohistiocytosis (HLH)- associated mutations affecting codon 65 of STXPB2, the gene encoding Munc18-2, a member proteins function in a dominant- of the SEC/MUNC18 family.