Investigating Predation in the Fossil Record: Modern Analogs
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Appendix 1 Table A1
OIK-00806 Kordas, R. L., Dudgeon, S., Storey, S., and Harley, C. D. G. 2014. Intertidal community responses to field-based experimental warming. – Oikos doi: 10.1111/oik.00806 Appendix 1 Table A1. Thermal information for invertebrate species observed on Salt Spring Island, BC. Species name refers to the species identified in Salt Spring plots. If thermal information was unavailable for that species, information for a congeneric from same region is provided (species in parentheses). Response types were defined as; optimum - the temperature where a functional trait is maximized; critical - the mean temperature at which individuals lose some essential function (e.g. growth); lethal - temperature where a predefined percentage of individuals die after a fixed duration of exposure (e.g., LT50). Population refers to the location where individuals were collected for temperature experiments in the referenced study. Distribution and zonation information retrieved from (Invertebrates of the Salish Sea, EOL) or reference listed in entry below. Other abbreviations are: n/g - not given in paper, n/d - no data for this species (or congeneric from the same geographic region). Invertebrate species Response Type Temp. Medium Exposure Population Zone NE Pacific Distribution Reference (°C) time Amphipods n/d for NE low- many spp. worldwide (Gammaridea) Pacific spp high Balanus glandula max HSP critical 33 air 8.5 hrs Charleston, OR high N. Baja – Aleutian Is, Berger and Emlet 2007 production AK survival lethal 44 air 3 hrs Vancouver, BC Liao & Harley unpub Chthamalus dalli cirri beating optimum 28 water 1hr/ 5°C Puget Sound, WA high S. CA – S. Alaska Southward and Southward 1967 cirri beating lethal 35 water 1hr/ 5°C survival lethal 46 air 3 hrs Vancouver, BC Liao & Harley unpub Emplectonema gracile n/d low- Chile – Aleutian Islands, mid AK Littorina plena n/d high Baja – S. -

Download Download
Appendix C: An Analysis of Three Shellfish Assemblages from Tsʼishaa, Site DfSi-16 (204T), Benson Island, Pacific Rim National Park Reserve of Canada by Ian D. Sumpter Cultural Resource Services, Western Canada Service Centre, Parks Canada Agency, Victoria, B.C. Introduction column sampling, plus a second shell data collect- ing method, hand-collection/screen sampling, were This report describes and analyzes marine shellfish used to recover seven shellfish data sets for investi- recovered from three archaeological excavation gating the siteʼs invertebrate materials. The analysis units at the Tseshaht village of Tsʼishaa (DfSi-16). reported here focuses on three column assemblages The mollusc materials were collected from two collected by the researcher during the 1999 (Unit different areas investigated in 1999 and 2001. The S14–16/W25–27) and 2001 (Units S56–57/W50– source areas are located within the village proper 52, S62–64/W62–64) excavations only. and on an elevated landform positioned behind the village. The two areas contain stratified cultural Procedures and Methods of Quantification and deposits dating to the late and middle Holocene Identification periods, respectively. With an emphasis on mollusc species identifica- The primary purpose of collecting and examining tion and quantification, this preliminary analysis the Tsʼishaa shellfish remains was to sample, iden- examines discarded shellfood remains that were tify, and quantify the marine invertebrate species collected and processed by the site occupants for each major stratigraphic layer. Sets of quantita- for approximately 5,000 years. The data, when tive information were compiled through out the reviewed together with the recovered vertebrate analysis in order to accomplish these objectives. -

JMS 70 1 031-041 Eyh003 FINAL
PHYLOGENY AND HISTORICAL BIOGEOGRAPHY OF LIMPETS OF THE ORDER PATELLOGASTROPODA BASED ON MITOCHONDRIAL DNA SEQUENCES TOMOYUKI NAKANO AND TOMOWO OZAWA Department of Earth and Planetary Sciences, Nagoya University, Nagoya 464-8602,Japan (Received 29 March 2003; accepted 6June 2003) ABSTRACT Using new and previously published sequences of two mitochondrial genes (fragments of 12S and 16S ribosomal RNA; total 700 sites), we constructed a molecular phylogeny for 86 extant species, covering a major part of the order Patellogastropoda. There were 35 lottiid, one acmaeid, five nacellid and two patellid species from the western and northern Pacific; and 34 patellid, six nacellid and three lottiid species from the Atlantic, southern Africa, Antarctica and Australia. Emarginula foveolata fujitai (Fissurellidae) was used as the outgroup. In the resulting phylogenetic trees, the species fall into two major clades with high bootstrap support, designated here as (A) a clade of southern Tethyan origin consisting of superfamily Patelloidea and (B) a clade of tropical Tethyan origin consisting of the Acmaeoidea. Clades A and B were further divided into three and six subclades, respectively, which correspond with geographical distributions of species in the following genus or genera: (AÍ) north eastern Atlantic (Patella ); (A2) southern Africa and Australasia ( Scutellastra , Cymbula-and Helcion)', (A3) Antarctic, western Pacific, Australasia ( Nacella and Cellana); (BÍ) western to northwestern Pacific (.Patelloida); (B2) northern Pacific and northeastern Atlantic ( Lottia); (B3) northern Pacific (Lottia and Yayoiacmea); (B4) northwestern Pacific ( Nipponacmea); (B5) northern Pacific (Acmaea-’ânà Niveotectura) and (B6) northeastern Atlantic ( Tectura). Approximate divergence times were estimated using geo logical events and the fossil record to determine a reference date. -

Seashore Beaty Box #007) Adaptations Lesson Plan and Specimen Information
Table of Contents (Seashore Beaty Box #007) Adaptations lesson plan and specimen information ..................................................................... 27 Welcome to the Seashore Beaty Box (007)! .................................................................................. 28 Theme ................................................................................................................................................... 28 How can I integrate the Beaty Box into my curriculum? .......................................................... 28 Curriculum Links to the Adaptations Lesson Plan ......................................................................... 29 Science Curriculum (K-9) ................................................................................................................ 29 Science Curriculum (10-12 Drafts 2017) ...................................................................................... 30 Photos: Unpacking Your Beaty Box .................................................................................................... 31 Tray 1: ..................................................................................................................................................... 31 Tray 2: .................................................................................................................................................... 31 Tray 3: .................................................................................................................................................. -

(Approx) Mixed Micro Shells (22G Bags) Philippines € 10,00 £8,64 $11,69 Each 22G Bag Provides Hours of Fun; Some Interesting Foraminifera Also Included
Special Price £ US$ Family Genus, species Country Quality Size Remarks w/o Photo Date added Category characteristic (€) (approx) (approx) Mixed micro shells (22g bags) Philippines € 10,00 £8,64 $11,69 Each 22g bag provides hours of fun; some interesting Foraminifera also included. 17/06/21 Mixed micro shells Ischnochitonidae Callistochiton pulchrior Panama F+++ 89mm € 1,80 £1,55 $2,10 21/12/16 Polyplacophora Ischnochitonidae Chaetopleura lurida Panama F+++ 2022mm € 3,00 £2,59 $3,51 Hairy girdles, beautifully preserved. Web 24/12/16 Polyplacophora Ischnochitonidae Ischnochiton textilis South Africa F+++ 30mm+ € 4,00 £3,45 $4,68 30/04/21 Polyplacophora Ischnochitonidae Ischnochiton textilis South Africa F+++ 27.9mm € 2,80 £2,42 $3,27 30/04/21 Polyplacophora Ischnochitonidae Stenoplax limaciformis Panama F+++ 16mm+ € 6,50 £5,61 $7,60 Uncommon. 24/12/16 Polyplacophora Chitonidae Acanthopleura gemmata Philippines F+++ 25mm+ € 2,50 £2,16 $2,92 Hairy margins, beautifully preserved. 04/08/17 Polyplacophora Chitonidae Acanthopleura gemmata Australia F+++ 25mm+ € 2,60 £2,25 $3,04 02/06/18 Polyplacophora Chitonidae Acanthopleura granulata Panama F+++ 41mm+ € 4,00 £3,45 $4,68 West Indian 'fuzzy' chiton. Web 24/12/16 Polyplacophora Chitonidae Acanthopleura granulata Panama F+++ 32mm+ € 3,00 £2,59 $3,51 West Indian 'fuzzy' chiton. 24/12/16 Polyplacophora Chitonidae Chiton tuberculatus Panama F+++ 44mm+ € 5,00 £4,32 $5,85 Caribbean. 24/12/16 Polyplacophora Chitonidae Chiton tuberculatus Panama F++ 35mm € 2,50 £2,16 $2,92 Caribbean. 24/12/16 Polyplacophora Chitonidae Chiton tuberculatus Panama F+++ 29mm+ € 3,00 £2,59 $3,51 Caribbean. -

UC Berkeley UC Berkeley Previously Published Works
UC Berkeley UC Berkeley Previously Published Works Title Twelve thousand recent patellogastropods from a northeastern Pacific latitudinal gradient. Permalink https://escholarship.org/uc/item/21h48289 Authors Kahanamoku, Sara Hull, Pincelli Lindberg, David et al. Publication Date 2018-01-09 DOI 10.1038/sdata.2017.197 Peer reviewed eScholarship.org Powered by the California Digital Library University of California www.nature.com/scientificdata OPEN Data Descriptor: Twelve thousand recent patellogastropods from a northeastern Pacific latitudinal gradient 13 2017 Received: June 1 2 1 2 1 3 Sara S. Kahanamoku , , Pincelli M. Hull , David R. Lindberg , Allison Y. Hsiang , , Accepted: 17 October 2017 4 2 Erica C. Clites & Seth Finnegan Published: 9 January 2018 Body size distributions can vary widely among communities, with important implications for ecological dynamics, energetics, and evolutionary history. Here we present a dataset of body size and shape for 12,035 extant Patellogastropoda (true limpet) specimens from the collections of the University of California Museum of Paleontology, compiled using a novel high-throughput morphometric imaging method. These specimens were collected over the past 150 years at 355 localities along a latitudinal gradient ranging from Alaska to Baja California, Mexico and are presented here with individual images, 2D outline coordinates, and 2D measurements of body size and shape. This dataset provides a resource for assemblage-scale macroecological questions and documents the size and diversity of recent patellogastropods in the northeastern Pacific. Design Type(s) observation design • image analysis objective Measurement Type(s) morphology Technology Type(s) digital camera Factor Type(s) geographic location Patellogastropoda • State of California • State of Baja California • State of Sample Characteristic(s) Washington • Mexico • State of Alaska • State of Oregon • Province of British Columbia 1 2 Yale University, Department of Geology & Geophysics, New Haven, CT 06511, USA. -

SPECIAL PUBLICATION 6 the Effects of Marine Debris Caused by the Great Japan Tsunami of 2011
PICES SPECIAL PUBLICATION 6 The Effects of Marine Debris Caused by the Great Japan Tsunami of 2011 Editors: Cathryn Clarke Murray, Thomas W. Therriault, Hideaki Maki, and Nancy Wallace Authors: Stephen Ambagis, Rebecca Barnard, Alexander Bychkov, Deborah A. Carlton, James T. Carlton, Miguel Castrence, Andrew Chang, John W. Chapman, Anne Chung, Kristine Davidson, Ruth DiMaria, Jonathan B. Geller, Reva Gillman, Jan Hafner, Gayle I. Hansen, Takeaki Hanyuda, Stacey Havard, Hirofumi Hinata, Vanessa Hodes, Atsuhiko Isobe, Shin’ichiro Kako, Masafumi Kamachi, Tomoya Kataoka, Hisatsugu Kato, Hiroshi Kawai, Erica Keppel, Kristen Larson, Lauran Liggan, Sandra Lindstrom, Sherry Lippiatt, Katrina Lohan, Amy MacFadyen, Hideaki Maki, Michelle Marraffini, Nikolai Maximenko, Megan I. McCuller, Amber Meadows, Jessica A. Miller, Kirsten Moy, Cathryn Clarke Murray, Brian Neilson, Jocelyn C. Nelson, Katherine Newcomer, Michio Otani, Gregory M. Ruiz, Danielle Scriven, Brian P. Steves, Thomas W. Therriault, Brianna Tracy, Nancy C. Treneman, Nancy Wallace, and Taichi Yonezawa. Technical Editor: Rosalie Rutka Please cite this publication as: The views expressed in this volume are those of the participating scientists. Contributions were edited for Clarke Murray, C., Therriault, T.W., Maki, H., and Wallace, N. brevity, relevance, language, and style and any errors that [Eds.] 2019. The Effects of Marine Debris Caused by the were introduced were done so inadvertently. Great Japan Tsunami of 2011, PICES Special Publication 6, 278 pp. Published by: Project Designer: North Pacific Marine Science Organization (PICES) Lori Waters, Waters Biomedical Communications c/o Institute of Ocean Sciences Victoria, BC, Canada P.O. Box 6000, Sidney, BC, Canada V8L 4B2 Feedback: www.pices.int Comments on this volume are welcome and can be sent This publication is based on a report submitted to the via email to: [email protected] Ministry of the Environment, Government of Japan, in June 2017. -

Lottia Pelta Class: Gastropoda, Patellogastropoda
Phylum: Mollusca Lottia pelta Class: Gastropoda, Patellogastropoda Order: The shield, or helmet limpet Family: Lottioidea, Lottiidae Taxonomy: A major systematic revision of (Sorensen and Lindberg 1991). May be fouled the northeastern Pacific limpet fauna was with a sabellid (Kuris and Culver 1999). undertaken by MacLean in 1966. Notoac- Interior: Blue gray to white, with mea was at first considered a subgenus and subapical brown spot (fig 3), and horseshoe- then later a full genus (MacLean 1969). Col- shaped muscle scar joined by a thin, faint line lisella was synonymized with Lottia, and lat- (fig. 3) (Keen and Coan 1974). Uses suction er Notoacmea was replaced with Tectura to attach to substratum, as well as a glue that (Lindberg 2007). The current practice in may be helpful in maintaining a seal around The Light and Smith Manual is to use only the edge of their feet on irregular surfaces Acmaea and Lottia to describe Pacific North- (Smith 1991). west species (Lindberg 2007). Possible Misidentifications Description Many species of limpets of the family Size: 25mm (Brusca and Brusca 1978); can Acmaeidae occur on our coast, but only about reach 40 mm farther north (Kozloff 1974b four are found in estuarine conditions. Lottia Yanes and Tyler 2009); illustrated specimen, scutum (=Notoacmaea), which, like Lottia pel- 32.5 mm. ta, have a horseshoe-shaped muscle scar on Color: Extremely variable dependent on the shell interior, joined by a thin curved line, substrata (Sorensen and Lindberg 1991); and various coloration, but not pink-rayed or called the brown and white shield limpet by white. These two genera differ in that L. -

Comparing the Mineralization of Two Limpet Species Lottia Pelta and Lottia Digitalis Between Species and Intertidal Location
Comparing the Mineralization of Two Limpet Species Lottia pelta and Lottia digitalis Between Species and Intertidal Location Leo MacLeod1 1Friday Harbor Laboratories, University of Washington Contact: Leo MacLeod Marine Biology, College of the Environment University of Washington Seattle, WA 98195 [email protected] Keywords: Limpet, CT scan, morphology, Lottia digitalis, Lottia pelta Abstract Intertidal gastropods have a variety of behavioral and physical adaptations to help them survive in the environment. One of the most obvious is the evolution of an outer shell for defense, which can protect organisms from dangers such as predators, as well as heat and wave force. The limpet species Lottia pelta and Lottia digitalis are two limpets with different external shell morphologies that live in overlapping habitats in the intertidal environment. In this study I aim to compare whether the visibly different shell morphological traits extend to the amount and distribution of mineralization within the shell and if this changes by tidal height. To do this I CT- scanned 31 limpet shells with phantoms of a known density to map the actual distribution of material densities. I found that while the overall density of each shell across species and intertidal height were not significantly different, the distribution of density differed for L. digitalis in low intertidal vs every other treatment. This difference that sets the low intertidal L. digitalis apart from the rest could be a result of the increased need for defense in a region that is often submerged and vulnerable to predation. Introduction The study of gastropod morphology has found many causes of variation in the size and shape of shells that tie back to the phenotypic plasticity within certain species. -
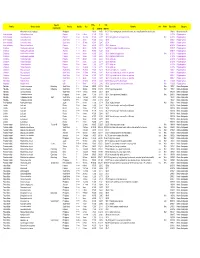
Approx) (Approx) Mixed Micro Shells (22G Bags) Philippines € 10,00 £8,56 $11,71 Each 22G Bag Provides Hours of Fun; Some Interesting Foraminifera Also Included
Special Price £ US$ Family Genus, species Country Quality Size Remarks w/o Photo Date added Category characteristic (€) (approx) (approx) Mixed micro shells (22g bags) Philippines € 10,00 £8,56 $11,71 Each 22g bag provides hours of fun; some interesting Foraminifera also included. 17/06/21 Mixed micro shells Ischnochitonidae Callistochiton pulchrior Panama F+++ 89mm € 1,80 £1,54 $2,11 21/12/16 Polyplacophora Ischnochitonidae Chaetopleura lurida Panama F+++ 2022mm € 3,00 £2,57 $3,51 Hairy girdles, beautifully preserved. Web 24/12/16 Polyplacophora Ischnochitonidae Ischnochiton textilis South Africa F+++ 30mm+ € 4,00 £3,42 $4,68 30/04/21 Polyplacophora Ischnochitonidae Ischnochiton textilis South Africa F+++ 27.9mm € 2,80 £2,40 $3,28 30/04/21 Polyplacophora Ischnochitonidae Stenoplax limaciformis Panama F+++ 16mm+ € 6,50 £5,57 $7,61 Uncommon. 24/12/16 Polyplacophora Chitonidae Acanthopleura gemmata Philippines F+++ 25mm+ € 2,50 £2,14 $2,93 Hairy margins, beautifully preserved. 04/08/17 Polyplacophora Chitonidae Acanthopleura gemmata Australia F+++ 25mm+ € 2,60 £2,23 $3,05 02/06/18 Polyplacophora Chitonidae Acanthopleura granulata Panama F+++ 41mm+ € 4,00 £3,42 $4,68 West Indian 'fuzzy' chiton. Web 24/12/16 Polyplacophora Chitonidae Acanthopleura granulata Panama F+++ 32mm+ € 3,00 £2,57 $3,51 West Indian 'fuzzy' chiton. 24/12/16 Polyplacophora Chitonidae Chiton tuberculatus Panama F+++ 44mm+ € 5,00 £4,28 $5,86 Caribbean. 24/12/16 Polyplacophora Chitonidae Chiton tuberculatus Panama F++ 35mm € 2,50 £2,14 $2,93 Caribbean. 24/12/16 Polyplacophora Chitonidae Chiton tuberculatus Panama F+++ 29mm+ € 3,00 £2,57 $3,51 Caribbean. -

Common Sea Life of Southeastern Alaska a Field Guide by Aaron Baldwin & Paul Norwood
Common Sea Life of Southeastern Alaska A field guide by Aaron Baldwin & Paul Norwood All pictures taken by Aaron Baldwin Last update 08/15/2015 unless otherwise noted. [email protected] Table of Contents Introduction ….............................................................…...2 Acknowledgements Exploring SE Beaches …………………………….….. …...3 It would be next to impossible to thanks everyone who has helped with Sponges ………………………………………….…….. …...4 this project. Probably the single-most important contribution that has been made comes from the people who have encouraged it along throughout Cnidarians (Jellyfish, hydroids, corals, the process. That is why new editions keep being completed! sea pens, and sea anemones) ……..........................…....8 First and foremost I want to thanks Rich Mattson of the DIPAC Macaulay Flatworms ………………………….………………….. …..21 salmon hatchery. He has made this project possible through assistance in obtaining specimens for photographs and for offering encouragement from Parasitic worms …………………………………………….22 the very beginning. Dr. David Cowles of Walla Walla University has Nemertea (Ribbon worms) ………………….………... ….23 generously donated many photos to this project. Dr. William Bechtol read Annelid (Segmented worms) …………………………. ….25 through the previous version of this, and made several important suggestions that have vastly improved this book. Dr. Robert Armstrong Mollusks ………………………………..………………. ….38 hosts the most recent edition on his website so it would be available to a Polyplacophora (Chitons) ……………………. -

INTERTIDAL LIMPETS of the FIDALGO BAY and CHERRY POINT AQUATIC RESERVES* Michael Kyte, Spring 2015 1
INTERTIDAL LIMPETS OF THE FIDALGO BAY AND CHERRY POINT AQUATIC RESERVES* Michael Kyte, Spring 2015 1. a. Shell margins nearly parallel, aperture elongated; color mostly dark brown, with light markings; length up to 1.2 cm; on leaves of native eelgrass, Zostera marina. Lottia alveus (L. parallela) Eelgrass Limpet b. Shell margins curving outward, outline oval.------------------2 2. a. Shell apex near or at one end; prominent radial ribs; length up to 3 cm; zone: upper to mid ITZ with littorine snails, Cthamalus dalli (Brown Barnacle), and Balanus glandula (Acorn Barnacle). Lottia digitalis Ribbed Limpet b. Apex not near or at one end; without prominent ribs; if ribs present, low, broad.-----------3 3. a. Height usually not more than one-third width, relatively flat when compared to other limpets; color brownish, olive-green, or greenish, usually spotted, streaked, or checkered with white; length sometimes exceeding 5 cm; common in mid- and low ITZ. Tectura scutum Plate Limpet b. Height usually more than one-third the width, relatively higher than T. scutum; color mostly uniformly brown, olive, or gray, but sometimes with a few white spots; length sometimes exceeding 3 cm.------------------- 4 Note: Published identification keys to limpets (e.g., Marine Invertebrates of the Pacific Northwest by Eugene Kozloff) rely heavily on interior markings of shells from which the animal has been removed. The following key assumes that only live animals resting on or attached to substrate will be examined and only the exterior of shells will be visible. Limpets Page 2 4. a. Apex at the end of the first one-third, often inclined slightly forward; shell appears “inflated” or bulging, smooth without ribs or wavy margin; color greenish brown, or olive- green with tan or white speckles, usually speckled and banded brown; length up to about 5 cm; largest limpet in mid to upper ITZ.