Redalyc.Towards an Understanding of the Interactions of Trypanosoma
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Trypanosoma Cruzi Immune Response Modulation Decreases Microbiota in Rhodnius Prolixus Gut and Is Crucial for Parasite Survival and Development
Trypanosoma cruzi Immune Response Modulation Decreases Microbiota in Rhodnius prolixus Gut and Is Crucial for Parasite Survival and Development Daniele P. Castro1*, Caroline S. Moraes1, Marcelo S. Gonzalez2, Norman A. Ratcliffe1, Patrı´cia Azambuja1,3, Eloi S. Garcia1,3 1 Laborato´rio de Bioquı´mica e Fisiologia de Insetos, Instituto Oswaldo Cruz, Fundac¸a˜o Oswaldo Cruz (Fiocruz), Rio de Janeiro, Rio de Janeiro, Brazil, 2 Laborato´rio de Biologia de Insetos, Departamento de Biologia Geral, Instituto de Biologia, Universidade Federal Fluminense (UFF) Nitero´i, Rio de Janeiro, Brazil, 3 Departamento de Entomologia Molecular, Instituto Nacional de Entomologia Molecular (INCT-EM), Rio de Janeiro, Rio de Janeiro, Brazil Abstract Trypanosoma cruzi in order to complete its development in the digestive tract of Rhodnius prolixus needs to overcome the immune reactions and microbiota trypanolytic activity of the gut. We demonstrate that in R. prolixus following infection with epimastigotes of Trypanosoma cruzi clone Dm28c and, in comparison with uninfected control insects, the midgut contained (i) fewer bacteria, (ii) higher parasite numbers, and (iii) reduced nitrite and nitrate production and increased phenoloxidase and antibacterial activities. In addition, in insects pre-treated with antibiotic and then infected with Dm28c, there were also reduced bacteria numbers and a higher parasite load compared with insects solely infected with parasites. Furthermore, and in contrast to insects infected with Dm28c, infection with T. cruzi Y strain resulted in a slight decreased numbers of gut bacteria but not sufficient to mediate a successful parasite infection. We conclude that infection of R. prolixus with the T. cruzi Dm28c clone modifies the host gut immune responses to decrease the microbiota population and these changes are crucial for the parasite development in the insect gut. -

When Hiking Through Latin America, Be Alert to Chagas' Disease
When Hiking Through Latin America, Be Alert to Chagas’ Disease Geographical distribution of main vectors, including risk areas in the southern United States of America INTERNATIONAL ASSOCIATION 2012 EDITION FOR MEDICAL ASSISTANCE For updates go to www.iamat.org TO TRAVELLERS IAMAT [email protected] www.iamat.org @IAMAT_Travel IAMATHealth When Hiking Through Latin America, Be Alert To Chagas’ Disease COURTESY ENDS IN DEATH segment upwards, releases a stylet with fine teeth from the proboscis and Valle de los Naranjos, Venezuela. It is late afternoon, the sun is sinking perforates the skin. A second stylet, smooth and hollow, taps a blood behind the mountains, bringing the first shadows of evening. Down in the vessel. This feeding process lasts at least twenty minutes during which the valley a campesino is still tilling the soil, and the stillness of the vinchuca ingests many times its own weight in blood. approaching night is broken only by a light plane, a crop duster, which During the feeding, defecation occurs contaminating the bite wound periodically flies overhead and disappears further down the valley. with feces which contain parasites that the vinchuca ingested during a Bertoldo, the pilot, is on his final dusting run of the day when suddenly previous bite on an infected human or animal. The irritation of the bite the engine dies. The world flashes before his eyes as he fights to clear the causes the sleeping victim to rub the site with his or her fingers, thus last row of palms. The old duster rears up, just clipping the last trees as it facilitating the introduction of the organisms into the bloodstream. -
A New Species of Rhodnius from Brazil (Hemiptera, Reduviidae, Triatominae)
A peer-reviewed open-access journal ZooKeys 675: 1–25A new (2017) species of Rhodnius from Brazil (Hemiptera, Reduviidae, Triatominae) 1 doi: 10.3897/zookeys.675.12024 RESEARCH ARTICLE http://zookeys.pensoft.net Launched to accelerate biodiversity research A new species of Rhodnius from Brazil (Hemiptera, Reduviidae, Triatominae) João Aristeu da Rosa1, Hernany Henrique Garcia Justino2, Juliana Damieli Nascimento3, Vagner José Mendonça4, Claudia Solano Rocha1, Danila Blanco de Carvalho1, Rossana Falcone1, Maria Tercília Vilela de Azeredo-Oliveira5, Kaio Cesar Chaboli Alevi5, Jader de Oliveira1 1 Faculdade de Ciências Farmacêuticas, Universidade Estadual Paulista “Júlio de Mesquita Filho” (UNESP), Araraquara, SP, Brasil 2 Departamento de Vigilância em Saúde, Prefeitura Municipal de Paulínia, SP, Brasil 3 Instituto de Biologia, Universidade Estadual de Campinas (UNICAMP), Campinas, SP, Brasil 4 Departa- mento de Parasitologia e Imunologia, Universidade Federal do Piauí (UFPI), Teresina, PI, Brasil 5 Instituto de Biociências, Letras e Ciências Exatas, Universidade Estadual Paulista “Júlio de Mesquita Filho” (UNESP), São José do Rio Preto, SP, Brasil Corresponding author: João Aristeu da Rosa ([email protected]) Academic editor: G. Zhang | Received 31 January 2017 | Accepted 30 March 2017 | Published 18 May 2017 http://zoobank.org/73FB6D53-47AC-4FF7-A345-3C19BFF86868 Citation: Rosa JA, Justino HHG, Nascimento JD, Mendonça VJ, Rocha CS, Carvalho DB, Falcone R, Azeredo- Oliveira MTV, Alevi KCC, Oliveira J (2017) A new species of Rhodnius from Brazil (Hemiptera, Reduviidae, Triatominae). ZooKeys 675: 1–25. https://doi.org/10.3897/zookeys.675.12024 Abstract A colony was formed from eggs of a Rhodnius sp. female collected in Taquarussu, Mato Grosso do Sul, Brazil, and its specimens were used to describe R. -

Vectors of Chagas Disease, and Implications for Human Health1
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Denisia Jahr/Year: 2006 Band/Volume: 0019 Autor(en)/Author(s): Jurberg Jose, Galvao Cleber Artikel/Article: Biology, ecology, and systematics of Triatominae (Heteroptera, Reduviidae), vectors of Chagas disease, and implications for human health 1095-1116 © Biologiezentrum Linz/Austria; download unter www.biologiezentrum.at Biology, ecology, and systematics of Triatominae (Heteroptera, Reduviidae), vectors of Chagas disease, and implications for human health1 J. JURBERG & C. GALVÃO Abstract: The members of the subfamily Triatominae (Heteroptera, Reduviidae) are vectors of Try- panosoma cruzi (CHAGAS 1909), the causative agent of Chagas disease or American trypanosomiasis. As important vectors, triatomine bugs have attracted ongoing attention, and, thus, various aspects of their systematics, biology, ecology, biogeography, and evolution have been studied for decades. In the present paper the authors summarize the current knowledge on the biology, ecology, and systematics of these vectors and discuss the implications for human health. Key words: Chagas disease, Hemiptera, Triatominae, Trypanosoma cruzi, vectors. Historical background (DARWIN 1871; LENT & WYGODZINSKY 1979). The first triatomine bug species was de- scribed scientifically by Carl DE GEER American trypanosomiasis or Chagas (1773), (Fig. 1), but according to LENT & disease was discovered in 1909 under curi- WYGODZINSKY (1979), the first report on as- ous circumstances. In 1907, the Brazilian pects and habits dated back to 1590, by physician Carlos Ribeiro Justiniano das Reginaldo de Lizárraga. While travelling to Chagas (1879-1934) was sent by Oswaldo inspect convents in Peru and Chile, this Cruz to Lassance, a small village in the state priest noticed the presence of large of Minas Gerais, Brazil, to conduct an anti- hematophagous insects that attacked at malaria campaign in the region where a rail- night. -

The Occurrence of Rhodnius Prolixus Stal, 1859, Naturally Infected By
Mem Inst Oswaldo Cruz, Rio de Janeiro, Vol. 93(2): 141-143, Mar./Apr. 1998 141 The studied area, Granja Florestal, Teresópolis, RESEARCH NOTE can be characterized as a secondary rain forest with poor human dwellings on the forests borders. The local population live basically on small agricul- The Occurrence of ture and hunting. Weekly searches were performed between September and March (1994-95) and in- Rhodnius prolixus Stal, 1859, cluded palm-trees, bracts of pteridophyta, bird and Naturally Infected by mammal nests, leafages and bromeliaceae. The collected insects were maintained in glass Trypanosoma cruzi in the flasks, fed through a membrane (ES Garcia et al. State of Rio de Janeiro, 1975 Rev Brasil Biol 35: 207-210) and the nymphs were allowed to moult. Seven isolates of T. cruzi Brazil (Hemiptera, were obtained through inoculation of swiss mice with the feces of the infected bugs. Axenic me- Reduviidae, Triatominae) dium derived metacyclic forms (105) from each Ana Paula Pinho, Teresa Cristina M isolate and were intraperitoneally inoculated in five swiss outbred mice weighing 18-20g. No mortal- Gonçalves*, Regina Helena ity occurred and only rare flagellates during a 2-3 Mangia**, Nédia S Nehme Russell**, day period could be observed in the fresh blood + Ana Maria Jansen/ preparations examined every other day, during two Departmento de Protozoologia *Departmento de months. Furthermore, the experimental infection Entomologia **Departmento de Bioquímica e by the isolates was stable as confirmed by the posi- Biologia Molecular, Instituto Oswaldo Cruz, Av. tive hemocultures made three months after the in- Brasil 4365, 21045-900 Rio de Janeiro, RJ, Brasil oculation. -

Disintegrins from Hematophagous Sources
Toxins 2012, 4, 296-322; doi:10.3390/toxins4050296 OPEN ACCESS toxins ISSN 2072-6651 www.mdpi.com/journal/toxins Review Disintegrins from Hematophagous Sources Teresa C. F. Assumpcao *, José M. C. Ribeiro * and Ivo M. B. Francischetti * Vector Biology Section, Laboratory of Malaria Vector Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD 20852, USA * Authors to whom correspondence should be addressed; E-Mails: [email protected] (T.C.F.A.); [email protected] (J.M.C.R.); [email protected] (I.M.B.F.) Received: 23 February 2012; in revised form: 12 April 2012 / Accepted: 13 April 2012 / Published: 26 April 2012 Abstract: Bloodsucking arthropods are a rich source of salivary molecules (sialogenins) which inhibit platelet aggregation, neutrophil function and angiogenesis. Here we review the literature on salivary disintegrins and their targets. Disintegrins were first discovered in snake venoms, and were instrumental in our understanding of integrin function and also for the development of anti-thrombotic drugs. In hematophagous animals, most disintegrins described so far have been discovered in the salivary gland of ticks and leeches. A limited number have also been found in hookworms and horseflies, and none identified in mosquitoes or sand flies. The vast majority of salivary disintegrins reported display a RGD motif and were described as platelet aggregation inhibitors, and few others as negative modulator of neutrophil or endothelial cell functions. This notably low number of reported disintegrins is certainly an underestimation of the actual complexity of this family of proteins in hematophagous secretions. Therefore an algorithm was created in order to identify the tripeptide motifs RGD, KGD, VGD, MLD, KTS, RTS, WGD, or RED (flanked by cysteines) in sialogenins deposited in GenBank database. -

Oviposition in the Blood-Sucking Insect Rhodnius Prolixus Is Modulated by Host Odors Fabio Guidobaldi1,2* and Pablo G Guerenstein1,2
Guidobaldi and Guerenstein Parasites & Vectors (2015) 8:265 DOI 10.1186/s13071-015-0867-5 RESEARCH Open Access Oviposition in the blood-sucking insect Rhodnius prolixus is modulated by host odors Fabio Guidobaldi1,2* and Pablo G Guerenstein1,2 Abstract Background: Triatomine bugs are blood-sucking insects, vectors of Chagas disease. Despite their importance, their oviposition behavior has received relatively little attention. Some triatomines including Rhodnius prolixus stick their eggs to a substrate. It is known that mechanical cues stimulate oviposition in this species. However, it is not clear if chemical signals play a role in this behavior. We studied the role of host cues, including host odor, in the oviposition behavior of the triatomine R. prolixus. Methods: Tests were carried out in an experimental arena and stimuli consisted of a mouse or hen feathers. The number of eggs laid and the position of those eggs with respect to the stimulus source were recorded. Data were analyzed using the Mann-Whitney and Kruskal-Wallis tests. Results: Both a mouse and hen feathers stimulated oviposition. In addition, hen feathers evoked a particular spatial distribution of eggs that was not observed in the case of mouse. Conclusions: We propose that volatile chemical cues from the host play a role in the oviposition behavior of triatomines that stick their eggs. Thus, host odor would stimulate and spatially guide oviposition. Keywords: Oviposition stimulation, Spatial distribution of eggs, Chagas disease, Triatomine, Hematophagous insect, Insect control, Insect monitoring Background In those cases in which optimal oviposition sites are The selection of sites for oviposition is a critical factor rare or difficult to find, female’s fitness would increase if for the survival of the offspring of an individual. -

Universidade Do Estado Da Bahia Anais Da Xxiii Jornada De Iniciação
UNIVERSIDADE DO ESTADO DA BAHIA ANAIS DA XXIII JORNADA DE INICIAÇÃO CIENTÍFICA “UNIVERSIDADE PÚBLICA E GRATUITA: RESISTÊNCIA, PRODUÇÃO CIENTÍFICA E TRANSFORMAÇÃO SOCIAL.” Salvador, 14 a 16 de outubro de 2019. FICHA CATALOGRÁFICA Sistema de Bibliotecas da UNEB Biblioteca Edivaldo Machado Boaventura Jornada de Iniciação Científica da UNEB (23.: 2019: Salvador, BA) Anais [da] / XXIII Jornada de Iniciação Científica da UNEB: Universidade Pública e Gratuita: Resistência, Produção Científica E Transformação Social, Salvador de 14 a 16 de outubro de 2019. Salvador: EDUNEB, 2019. 635p. ISSN : 2237-6895. 1. Ensino superior - Pesquisa - Brasil - Congressos. 2. Pesquisa - Bahia - Congressos. I. Universidade do Estado da Bahia - Congressos. CDD: 378.0072 UNIVERSIDADE DO ESTADO DA BAHIA REITORIA JOSÉ BITES DE CARVALHO VICE-REITORIA MARCELO DUARTE DANTAS ÁVILA CHEFIA DE GABINETE (CHEGAB) HILDA SILVA FERREIRA PRÓ-REITORIA DE ENSINO DE GRADUAÇÃO (PROGRAD) ELIENE MARIA DA SILVA PRÓ-REITORIA DE PESQUISA E ENSINO DE PÓS-GRADUAÇÃO (PPG) TÂNIA MARIA HETKOWSKI PRÓ-REITORIA DE EXTENSÃO (PROEX) ADRIANA SANTOS MARMORE LIMA PRÓ-REITORIA DE ADMINISTRAÇÃO (PROAD) DANIEL DE CERQUEIRA GÓES PRÓ-REITORIA DE PLANEJAMENTO (PROPLAN) IZA ANGELICA CARVALHO DA SILVA PRÓ-REITORIA DE GESTÃO E DESENVOLVIMENTO (PGDP) LILIAN DA ENCARNAÇÃO CONCEIÇÃO PRÓ-REITORIA DE AÇÕES AFIRMATIVA (PROAF) AMELIA TEREZA SANTA ROSA MARAUX PRÓ-REITORIA DE ASSISTÊNCIA ESTUDANTIL (PRAES) ELIVÂNIA REIS DE ANDRADE ALVES PRÓ-REITORIA DE INFRAESTRUTURA (PROINFRA) FAUSTO FERREIRA COSTA GUIMARÃES UNIDADE -

Distribution and Ecological Aspects of Rhodnius Pallescens in Costa Rica
Mem Inst Oswaldo Cruz, Rio de Janeiro, Vol. 101(1): 75-79, February 2006 75 Distribution and ecological aspects of Rhodnius pallescens in Costa Rica and Nicaragua and their epidemiological implications Rodrigo Zeledón/+, Francisca Marín*, Nidia Calvo**, Emperatriz Lugo*, Sonia Valle* Escuela de Medicina Veterinaria, Universidad Nacional, Apartado Postal 86, Heredia, Costa Rica *Programa de Enfermedad de Chagas, Ministerio de Salud, Managua, Nicaragua **Instituto Costarricense de Investigación y Enseñanza en Nutrición y Salud, Tres Ríos, Cartago, Costa Rica In light of the Central American Initiative for the control of Chagas disease, efforts were made on the part of Costa Rican and Nicaraguan teams, working separately, to determine the present status of Rhodnius pallescens in areas close to the common border of the two countries, where the insect has appeared within the last few years. The opportunity was also used to establish whether R. prolixus, a vector present in some areas of Nicaragua, has been introduced in recent years into Costa Rica with Nicaraguan immigrants. It became evident that wild adults of R. pallescens are common visitors to houses in different towns of a wide area characterized as a humid, warm lowland, on both sides of the frontier. Up to the present, this bug has been able to colonize a small proportion of human dwellings only on the Nicaraguan side. There was strong evidence that the visitation of the adult bug to houses is related to the attraction of this species to electric lights. There were no indications of the presence of R. prolixus either in Nicaragua or in Costa Rica in this area of the Caribbean basin. -

Mitteilungen Der Dgaae 18
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/251383302 “Living Syringes”: Use of Hematophagous Bugs as Blood Samplers from Small and Wild Animals Article · May 2011 DOI: 10.1007/978-3-642-19382-8_11 CITATIONS READS 10 314 3 authors: André Stadler Christian Karl Meiser Alpenzoo Ruhr-Universität Bochum 20 PUBLICATIONS 41 CITATIONS 14 PUBLICATIONS 164 CITATIONS SEE PROFILE SEE PROFILE Guenter Schaub Ruhr-Universität Bochum 187 PUBLICATIONS 5,617 CITATIONS SEE PROFILE All content following this page was uploaded by Guenter Schaub on 14 December 2015. The user has requested enhancement of the downloaded file. HALLE (SAALE ) 2012 MITT . DTSCH . GES . ALL G . AN G EW . ENT . 18 “Living syringes”: use of triatomines as blood samplers from small and wild animals Günter A. Schaub1, Arne Lawrenz2 & André Stadler2 1Zoology/Parasitology Group, Ruhr-University Bochum 2Zoological Garden Wuppertal Zusammenfassung: Die Blutabnahme ist bei kleinen Tieren und Wildtieren sehr schwierig. Bei der konventionellen Entnahme werden die Tiere sehr gestresst und evtl. verletzt. Auch eine vorherige Narkose ist problematisch. Triatominen (Reduviidae, Hemiptera) sind die größten Blut saugenden Insekten und saugen an allen Endothermen, aber auch warmen Amphibien und Reptilien. Die fünf Nymphenstadien dieser Insekten nehmen – entsprechend ihrem Wachstum – zunehmend mehr Blut auf. Deshalb ist je nach erforderlicher Blutmenge beim Einsatz als „lebende Spritze“ ein entsprechendes Nymphenstadium einzusetzen. Das aufgenommene Blut wird im Magen gespeichert und durch den Entzug der wässrigen Blutbestandteile konzentriert, aber fast nicht verdaut. Das Blut kann direkt nach der Blutaufnahme der Raubwanze leicht mit einer Spritze aus dem Magen entnommen und zur Bestimmung der Blut- und physiologischen Parameter eingesetzt werden, außerdem zur Bestimmung von Hormon- und Antikörper-Konzentrationen sowie zum Nachweis von Parasiten. -
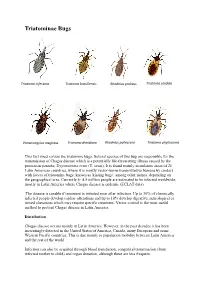
Triatominae Bugs
Triatominae Bugs Triatoma infestans Triatoma brasiliensis Rhodnius prolixus Triatoma sordida Panstrongylus megistus Triatoma dimidiata Rhodnius pallescens Triatoma phyllosoma This fact sheet covers the triatomine bugs. Several species of this bug are responsible for the transmission of Chagas disease which is a potentially life-threatening illness caused by the protozoan parasite, Trypanosoma cruzi (T. cruzi). It is found mainly in endemic areas of 21 Latin American countries, where it is mostly vector-borne transmitted to humans by contact with faeces of triatomine bugs, known as 'kissing bugs', among other names, depending on the geographical area. Currently 4- 4.5 million people are estimated to be infected worldwide, mostly in Latin America where Chagas disease is endemic (ECLAT data). The disease is curable if treatment is initiated soon after infection. Up to 30% of chronically infected people develop cardiac alterations and up to 10% develop digestive, neurological or mixed alterations which may require specific treatment. Vector control is the most useful method to prevent Chagas disease in Latin America. Distribution Chagas disease occurs mainly in Latin America. However, in the past decades it has been increasingly detected in the United States of America, Canada, many European and some Western Pacific countries. This is due mainly to population mobility between Latin America and the rest of the world. Infection can also be acquired through blood transfusion, congenital transmission (from infected mother to child) and organ donation, although these are less frequent. Transmission In Latin America, T. cruzi parasites are mainly transmitted by contact with the faeces of infected blood-sucking triatomine bugs. These bugs, vectors that carry the parasites, typically live in the cracks of poorly-constructed homes in rural or suburban areas. -

Proteases of Haematophagous Arthropod Vectors
Santiago et al. Parasites & Vectors (2017) 10:79 DOI 10.1186/s13071-017-2005-z REVIEW Open Access Proteases of haematophagous arthropod vectors are involved in blood-feeding, yolk formation and immunity - a review Paula Beatriz Santiago1, Carla Nunes de Araújo1,2, Flávia Nader Motta1,2, Yanna Reis Praça1,3, Sébastien Charneau4, Izabela M. Dourado Bastos1 and Jaime M. Santana1* Abstract Ticks, triatomines, mosquitoes and sand flies comprise a large number of haematophagous arthropods considered vectors of human infectious diseases. While consuming blood to obtain the nutrients necessary to carry on life functions, these insects can transmit pathogenic microorganisms to the vertebrate host. Among the molecules related to the blood-feeding habit, proteases play an essential role. In this review, we provide a panorama of proteases from arthropod vectors involved in haematophagy, in digestion, in egg development and in immunity. As these molecules act in central biological processes, proteases from haematophagous vectors of infectious diseases may influence vector competence to transmit pathogens to their prey, and thus could be valuable targets for vectorial control. Keywords: Proteases, Haematophagy, Digestion, Yolk formation, Immunity, Ticks, Triatomines, Mosquitoes Background leishmaniasis, malaria, sleeping sickness, lymphatic filaria- Haematophagous arthropod vectors are spread world- sis and onchocerciasis are all examples of vector-borne wide. They are of medical and veterinary importance diseases with global impact on morbidity and mortality since their blood-feeding habit provides a scenario for (Table 1) since they affect more than one billion individ- the transmission of a variety of pathogens, including uals and cause over one million deaths every year [2]. virus, bacteria, protozoans and helminths [1].