Crystals of Golden Proportions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Enigmatic Number E: a History in Verse and Its Uses in the Mathematics Classroom
To appear in MAA Loci: Convergence The Enigmatic Number e: A History in Verse and Its Uses in the Mathematics Classroom Sarah Glaz Department of Mathematics University of Connecticut Storrs, CT 06269 [email protected] Introduction In this article we present a history of e in verse—an annotated poem: The Enigmatic Number e . The annotation consists of hyperlinks leading to biographies of the mathematicians appearing in the poem, and to explanations of the mathematical notions and ideas presented in the poem. The intention is to celebrate the history of this venerable number in verse, and to put the mathematical ideas connected with it in historical and artistic context. The poem may also be used by educators in any mathematics course in which the number e appears, and those are as varied as e's multifaceted history. The sections following the poem provide suggestions and resources for the use of the poem as a pedagogical tool in a variety of mathematics courses. They also place these suggestions in the context of other efforts made by educators in this direction by briefly outlining the uses of historical mathematical poems for teaching mathematics at high-school and college level. Historical Background The number e is a newcomer to the mathematical pantheon of numbers denoted by letters: it made several indirect appearances in the 17 th and 18 th centuries, and acquired its letter designation only in 1731. Our history of e starts with John Napier (1550-1617) who defined logarithms through a process called dynamical analogy [1]. Napier aimed to simplify multiplication (and in the same time also simplify division and exponentiation), by finding a model which transforms multiplication into addition. -

Quartz Crystal Division of Seiko Instruments Inc
(1) Quartz Crystal Division of Seiko Instruments Inc. and affiliates, which is responsible for manufacturing the products described in this catalogue, holds ISO 9001 and ISO 14001 certification. (2) SII Crystal Technology Inc. Tochigi site holds IATF 16949 certification. Quartz Crystal Product Catalogue Electronic Components Sales Head Office 1-8, Nakase, Mihamaku, Chiba-shi, Chiba 261-8507, Japan Telephone:+81-43-211-1207 Facsimile:+81-43-211-8030 E-mail:[email protected] <Manufacturer> SII Crystal Technology Inc. 1110, Hirai-cho, Tochigi-shi, Tochigi 328-0054, Japan Released in February 2019 No.QTC2019EJ-02C1604 Creating Time - Optimizing Time - Enriching Time Seiko Instruments Inc. (SII), founded in 1937 as a member of the Seiko Group specializing in the manufacture of watches, has leveraged its core competency in high precision watches to create a wide range of new products and technologies. Over the years SII has developed high-precision processed parts and machine tools that pride themselves on their sub-micron processing capability, quartz crystals that came about as a result of our quartz watch R&D, and electronic components such as micro batteries. Optimizing our extensive experience and expertise, we have since diversified into such new fields as compact, lightweight, exceedingly quiet thermal printers, and inkjet printheads, a key component in wide format inkjet printers for corporate use. SII, in the years to come, will maintain an uncompromised dedication to its time-honored technologies and innovations of craftsmanship, miniaturization, and efficiency that meet the needs of our changing society and enrich the lives of those around us. SEIKO HOLDINGS GROUP 1881 1917 1983 1997 2007 K. -

WHAT IS...A Quasicrystal?, Volume 53, Number 8
?WHAT IS... a Quasicrystal? Marjorie Senechal The long answer is: no one is sure. But the short an- diagrams? The set of vertices of a Penrose tiling does— swer is straightforward: a quasicrystal is a crystal that was known before Shechtman’s discovery. But with forbidden symmetry. Forbidden, that is, by “The what other objects do, and how can we tell? The ques- Crystallographic Restriction”, a theorem that confines tion was wide open at that time, and I thought it un- the rotational symmetries of translation lattices in two- wise to replace one inadequate definition (the lattice) and three-dimensional Euclidean space to orders 2, 3, with another. That the commission still retains this 4, and 6. This bedrock of theoretical solid-state sci- definition today suggests the difficulty of the ques- ence—the impossibility of five-fold symmetry in crys- tion we deliberately but implicitly posed. By now a tals can be traced, in the mineralogical literature, back great many kinds of aperiodic crystals have been to 1801—crumbled in 1984 when Dany Shechtman, a grown in laboratories around the world; most of them materials scientist working at what is now the National are metals, alloys of two or three kinds of atoms—bi- Institute of Standards and Technology, synthesized nary or ternary metallic phases. None of their struc- aluminium-manganese crystals with icosahedral sym- tures has been “solved”. (For a survey of current re- metry. The term “quasicrystal”, hastily coined to label search on real aperiodic crystals see, for example, the such theretofore unthinkable objects, suggests the website of the international conference ICQ9, confusions that Shechtman’s discovery sowed. -

Bubble Raft Model for a Paraboloidal Crystal
Syracuse University SURFACE Physics College of Arts and Sciences 9-17-2007 Bubble Raft Model for a Paraboloidal Crystal Mark Bowick Department of Physics, Syracuse University, Syracuse, NY Luca Giomi Syracuse University Homin Shin Syracuse University Creighton K. Thomas Syracuse University Follow this and additional works at: https://surface.syr.edu/phy Part of the Physics Commons Recommended Citation Bowick, Mark; Giomi, Luca; Shin, Homin; and Thomas, Creighton K., "Bubble Raft Model for a Paraboloidal Crystal" (2007). Physics. 144. https://surface.syr.edu/phy/144 This Article is brought to you for free and open access by the College of Arts and Sciences at SURFACE. It has been accepted for inclusion in Physics by an authorized administrator of SURFACE. For more information, please contact [email protected]. Bubble Raft Model for a Paraboloidal Crystal Mark J. Bowick, Luca Giomi, Homin Shin, and Creighton K. Thomas Department of Physics, Syracuse University, Syracuse New York, 13244-1130 We investigate crystalline order on a two-dimensional paraboloid of revolution by assembling a single layer of millimeter-sized soap bubbles on the surface of a rotating liquid, thus extending the classic work of Bragg and Nye on planar soap bubble rafts. Topological constraints require crystalline configurations to contain a certain minimum number of topological defects such as disclinations or grain boundary scars whose structure is analyzed as a function of the aspect ratio of the paraboloid. We find the defect structure to agree with theoretical predictions and propose a mechanism for scar nucleation in the presence of large Gaussian curvature. Soft materials such as amphiphilic membranes, diblock any triangulation of M reads copolymers and colloidal emulsions can form ordered structures with a wide range of complex geometries and Q = X(6 ci)+ X (4 ci)=6χ , (1) − − topologies. -

Crystal Structures and Symmetry 1 Crystal Structures and Symmetry
PHYS 624: Crystal Structures and Symmetry 1 Crystal Structures and Symmetry Introduction to Solid State Physics http://www.physics.udel.edu/∼bnikolic/teaching/phys624/phys624.html PHYS 624: Crystal Structures and Symmetry 2 Translational Invariance • The translationally invariant nature of the periodic solid and the fact that the core electrons are very tightly bound at each site (so we may ignore their dynamics) makes approximate solutions to many-body problem ≈ 1021 atoms/cm3 (essentially, a thermodynamic limit) possible. Figure 1: The simplest model of a solid is a periodic array of valance orbitals embedded in a matrix of atomic cores. Solving the problem in one of the irreducible elements of the periodic solid (e.g., one of the spheres in the Figure), is often equivalent to solving the whole system. PHYS 624: Crystal Structures and Symmetry 3 From atomic orbitals to solid-state bands • If two orbitals are far apart, each orbital has a Hamiltonian H0 = εn, where n is the orbital occupancy ⇐ Ignoring the effects of electronic corre- lations (which would contribute terms proportional to n↑n↓). + +++ ... = Band E Figure 2: If we bring many orbitals into proximity so that they may exchange electrons (hybridize), then a band is formed centered around the location of the isolated orbital, and with width proportional to the strength of the hybridization PHYS 624: Crystal Structures and Symmetry 4 From atomic orbitals to solid-state bands • Real Life: Solids are composed of elements with multiple orbitals that produce multiple bonds. Now imagine what happens if we have several orbitals on each site (ie s,p, etc.), as we reduce the separation between the orbitals and increase their overlap, these bonds increase in width and may eventually overlap, forming bands. -

Crystal Symmetry Groups
X-Ray and Neutron Crystallography rational numbers is a group under Crystal Symmetry Groups multiplication, and both it and the integer group already discussed are examples of infinite groups because they each contain an infinite number of elements. ymmetry plays an important role between the integers obey the rules of In the case of a symmetry group, in crystallography. The ways in group theory: an element is the operation needed to which atoms and molecules are ● There must be defined a procedure for produce one object from another. For arrangeds within a unit cell and unit cells example, a mirror operation takes an combining two elements of the group repeat within a crystal are governed by to form a third. For the integers one object in one location and produces symmetry rules. In ordinary life our can choose the addition operation so another of the opposite hand located first perception of symmetry is what that a + b = c is the operation to be such that the mirror doing the operation is known as mirror symmetry. Our performed and u, b, and c are always is equidistant between them (Fig. 1). bodies have, to a good approximation, elements of the group. These manipulations are usually called mirror symmetry in which our right side ● There exists an element of the group, symmetry operations. They are com- is matched by our left as if a mirror called the identity element and de- bined by applying them to an object se- passed along the central axis of our noted f, that combines with any other bodies. -

Quasicrystals a New Kind of Symmetry Sandra Nair First, Definitions
Quasicrystals A new kind of symmetry Sandra Nair First, definitions ● A lattice is a poset in which every element has a unique infimum and supremum. For example, the set of natural numbers with the notion of ordering by magnitude (1<2). For our purposes, we can think of an array of atoms/molecules with a clear sense of assignment. ● A Bravais lattice is a discrete infinite array of points generated by linear integer combinations of 3 independent primitive vectors: {n1a1 + n2a2 + n3a3 | n1, n2, n3 ∈ Z}. ● Crystal structures = info of lattice points + info of the basis (primitive) vectors. ● Upto isomorphism of point groups (group of isometries leaving at least 1 fixed point), 14 different Bravais lattice structures possible in 3D. Now, crystals... ● Loosely speaking, crystals are molecular arrangements built out of multiple unit cells of one (or more) Bravais lattice structures. ● Crystallographic restriction theorem: The rotational symmetries of a discrete lattice are limited to 2-, 3-, 4-, and 6-fold. ● This leads us to propose a “functional” definition: A crystal is a material that has a discrete diffraction pattern, displaying rotational symmetries of orders 2, 3, 4 and 6. ● Note: Order 5 is a strictly forbidden symmetry → important for us. Tessellations aka tilings Now that we have diffraction patterns to work with, we consider the question of whether a lattice structure tiles or tessellates the plane. This is where the order of the symmetry plays a role. The crystals are special, as they display translational symmetries. As such, the tiling of their lattice structures (which we could see thanks to diffraction patterns) are periodic- they repeat at regular intervals. -
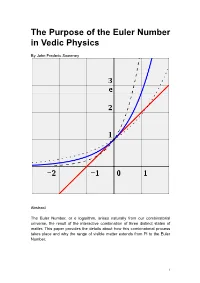
Euler Number in Vedic Physics
The Purpose of the Euler Number in Vedic Physics By John Frederic Sweeney Abstract The Euler Number, or e logarithm, arises naturally from our combinatorial universe, the result of the interactive combination of three distinct states of matter. This paper provides the details about how this combinatorial process takes place and why the range of visible matter extends from Pi to the Euler Number. 1 Table of Contents Introduction 3 Wikipedia on the Euler Number 5 Vedic Physics on the Euler Number 6 Conclusion 8 Bibliography 9 2 Introduction The author posted a few papers on Vixra during 2013 which made statements about Pi and the Euler Number that lacked support. This paper offers the support for those statements. Since the explanation is mired, twisted and difficult to follow, the author wished to make other aspects of Vedic Particle Physics clear. This paper begins with Wikipedia on Euler, then to the Vedic Explanation. This paper, along with Why Pi? Which explains the reason why Pi carries such importance in a combinatorial world, should make it easier for readers to grasp the fundamental concepts of Vedic Particle Physics. Essentially, three states of matter exist in a combinatorial world, the axes of the states run from Pi to the Euler number. The simple formula of n + 1 suggests Pascal’s Triangle or Mount Meru, and the author has published a paper on this theme about Clifford Algebras, suggesting them as a most useful tool in a combinatorial universe. Someone once said that only those with clear understanding can explain things clearly and simply to others. -

Multidisciplinary Design Project Engineering Dictionary Version 0.0.2
Multidisciplinary Design Project Engineering Dictionary Version 0.0.2 February 15, 2006 . DRAFT Cambridge-MIT Institute Multidisciplinary Design Project This Dictionary/Glossary of Engineering terms has been compiled to compliment the work developed as part of the Multi-disciplinary Design Project (MDP), which is a programme to develop teaching material and kits to aid the running of mechtronics projects in Universities and Schools. The project is being carried out with support from the Cambridge-MIT Institute undergraduate teaching programe. For more information about the project please visit the MDP website at http://www-mdp.eng.cam.ac.uk or contact Dr. Peter Long Prof. Alex Slocum Cambridge University Engineering Department Massachusetts Institute of Technology Trumpington Street, 77 Massachusetts Ave. Cambridge. Cambridge MA 02139-4307 CB2 1PZ. USA e-mail: [email protected] e-mail: [email protected] tel: +44 (0) 1223 332779 tel: +1 617 253 0012 For information about the CMI initiative please see Cambridge-MIT Institute website :- http://www.cambridge-mit.org CMI CMI, University of Cambridge Massachusetts Institute of Technology 10 Miller’s Yard, 77 Massachusetts Ave. Mill Lane, Cambridge MA 02139-4307 Cambridge. CB2 1RQ. USA tel: +44 (0) 1223 327207 tel. +1 617 253 7732 fax: +44 (0) 1223 765891 fax. +1 617 258 8539 . DRAFT 2 CMI-MDP Programme 1 Introduction This dictionary/glossary has not been developed as a definative work but as a useful reference book for engi- neering students to search when looking for the meaning of a word/phrase. It has been compiled from a number of existing glossaries together with a number of local additions. -

The Exponential Constant E
The exponential constant e mc-bus-expconstant-2009-1 Introduction The letter e is used in many mathematical calculations to stand for a particular number known as the exponential constant. This leaflet provides information about this important constant, and the related exponential function. The exponential constant The exponential constant is an important mathematical constant and is given the symbol e. Its value is approximately 2.718. It has been found that this value occurs so frequently when mathematics is used to model physical and economic phenomena that it is convenient to write simply e. It is often necessary to work out powers of this constant, such as e2, e3 and so on. Your scientific calculator will be programmed to do this already. You should check that you can use your calculator to do this. Look for a button marked ex, and check that e2 =7.389, and e3 = 20.086 In both cases we have quoted the answer to three decimal places although your calculator will give a more accurate answer than this. You should also check that you can evaluate negative and fractional powers of e such as e1/2 =1.649 and e−2 =0.135 The exponential function If we write y = ex we can calculate the value of y as we vary x. Values obtained in this way can be placed in a table. For example: x −3 −2 −1 01 2 3 y = ex 0.050 0.135 0.368 1 2.718 7.389 20.086 This is a table of values of the exponential function ex. -

Crystal Wst - Opaque
MELT & POUR CRYSTAL WST - OPAQUE THE IDEAL STARTING POINT FOR CREATING HANDMADE SOAPS, CREATE A WIDE RANGE OF BEAUTIFUL PRODUCTS. SIMPLY COMBINE COLOUR AND FRAGRANCE TO TAILOR YOUR SOAPS. Vegetable based melt and pour soap base, used for manufacture of opaque soap bars. Crystal WST has great REMOVE foaming qualities and good moisturising from a very high glycerine content which provides a pleasant skin feel. Superior colour stability even if kept at a a high temperature for extended periods. Neutral odour provides excellent fragrance lift. Crystal soap bases are ideal for manufacturers, brands and home hobbyists. Simply Melt, Mix, Pour, Create. CUT Vegetable based. Good foaming. Good moisturising from a very high glycerine content MELT which provides a pleasant skin feel. Unlike other soap bases, our base contains NO Triethanolamine Superior colour stability even if kept at a high temperature for extended periods. Neutral odour provides excellent fragrance lift Suitable for slicing or moulding. With added Titanium Dioxide as an opacifier. POUR Suitable for slicing and moulding. MADE IN ENGLAND GUIDE RECIPE Here we show you how to create a simple Crystal WST - Opaque Soap Bar. To create your very own set of hand made glycerin soap bars, here is what you’ll need: Crystal WST - Opaque - Slice into meltable chunks Fragrance if you wish Add dried Botanicals if you wish A soap mould and slicer Microwave / Double boiler Open / Cut - Simply open the packaging, safely cut the Crystal soap base into smaller cubes, doing this will make the soap easier to handle and melt. Melt - Gradually heat the soap base ensuring that the soap melts evenly and does not boil and burn. -

Activity 21: Cleavage and Fracture Maine Geological Survey
Activity 21: Cleavage and Fracture Maine Geological Survey Objectives: Students will recognize the difference between cleavage and fracture; they will become familiar with planes of cleavage, and will use a mineral's "habit of breaking" as an aid to identifying common minerals. Time: This activity is intended to take one-half (1/2) period to discuss cleavage planes and types and one (1) class period to do the activity. Background: Cleavage is the property of a mineral that allows it to break smoothly along specific internal planes (called cleavage planes) when the mineral is struck sharply with a hammer. Fracture is the property of a mineral breaking in a more or less random pattern with no smooth planar surfaces. Since nearly all minerals have an orderly atomic structure, individual mineral grains have internal axes of length, width, and depth, related to the consistent arrangement of the atoms. These axes are reflected in the crystalline pattern in which the mineral grows and are present in the mineral regardless of whether or not the sample shows external crystal faces. The axes' arrangement, size, and the angles at which these axes intersect, all help determine, along with the strength of the molecular bonding in the given mineral, the degree of cleavage the mineral will exhibit. Many minerals, when struck sharply with a hammer, will break smoothly along one or more of these planes. The degree of smoothness of the broken surface and the number of planes along which the mineral breaks are used to describe the cleavage. The possibilities include the following. Number of Planes Degree of Smoothness One Two Three Perfect Good Poor Thus a mineral's cleavage may be described as perfect three plane cleavage, in which case the mineral breaks with almost mirror-like surfaces along the three dimensional axes; the mineral calcite exhibits such cleavage.