An Assessment of the Influence of Host Species, Age, and Thallus Part
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
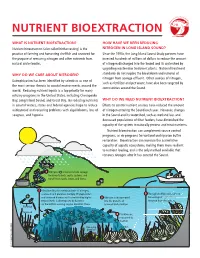
Nutrient Bioextraction L
soun nd d s sla tu i d g y n o NUTRIENT BIOEXTRACTION l WHAT IS NUTRIENT BIOEXTRACTION? HOW HAVE WE BEEN REDUCING Nutrient bioextraction (also called bioharvesting) is the NITROGEN IN LONG ISLAND SOUND? practice of farming and harvesting shellfish and seaweed for Since the 1990s, the Long Island Sound Study partners have the purpose of removing nitrogen and other nutrients from invested hundreds of millions of dollars to reduce the amount natural water bodies. of nitrogen discharged into the Sound and its watershed by upgrading wastewater treatment plants. National treatment WHY DO WE CARE ABOUT NITROGEN? standards do not require the breakdown and removal of nitrogen from sewage effluent. Other sources of nitrogen, Eutrophication has been identified by scientists as one of such as fertilizer and pet waste, have also been targeted by the most serious threats to coastal environments around the communities around the Sound. world. Reducing nutrient inputs is a top priority for many estuary programs in the United States, including Chesapeake Bay, Long Island Sound, and Great Bay. By reducing nutrients WHY DO WE NEED NUTRIENT BIOEXTRACTION? in coastal waters, states and federal agencies hope to reduce Efforts to control nutrient sources have reduced the amount widespread and recurring problems with algal blooms, loss of of nitrogen entering the Sound each year. However, changes seagrass, and hypoxia. in the Sound and its watershed, such as wetland loss and decreased populations of filter feeders, have diminished the capacity of the system to naturally process and treat nutrients. Nutrient bioextraction can complement source control programs, as do programs for wetland and riparian buffer restoration. -

Icelandic Geothermal Kelp – Specifications
Icelandic Geothermal Kelp – Specifications Laminaria digitata Certified 100% Organic PRODUCT DESCRIPTION Species Laminaria digitata Plant Part Milled Sea Vegetation (whole thallus) Processing Method Sustainable harvest, controlled geothermal low-temperature drying Country of Origin Iceland Primary Active Phytonutrients, Iodine and other micronutrients Recommended Daily Serving 50 milligrams* Particle Size Granules and Powder Color Green Aroma Mild marine odor Taste Salty Storage Dry area Shelf Life Best used within 60-months Packaging 25 kg. (55 lbs.); Multi-walled Kraft bag; easy-pour spout Certificates Certified 100% Organic by QAI; Certified Kosher by Star-K ANALYSIS Activity 2500-7500 ppm (0.25 – 0.75%) Iodine Moisture NMT 10% Test Methods Ash NMT 50% Lead NMT 5 ppm ICP-MS Inorganic Arsenic NMT 30 ppm IC-ICP-MS Cadmium NMT 1.5 ppm ICP-MS Mercury NMT 0.05 ppm ICP-MS Aerobic Plate Count <10,000 CFU/g FDA BAM 3 Total Coliform <1,000 CFU/g FDA BAM 4 Microbial E. coli N/D (<10 CFU/g) FDA BAM 4 Salmonella Negative (ND/25g) AOAC-989.09 Yeast/Mold <2,500 CFU/g each FDA BAM 18 Thorvin contains over 60 minerals, vitamins, amino acids, and beneficial phytonutrients. Thorvin is a 100% natural organic marine algae product; therefore, a specific laboratory analysis may vary from the typical analysis due to naturally occurring fluctuations in the sea plant. The information presented above is believed to be accurate and reliable; however, Thorvin, Inc. makes no warranty, either express or implied, and assumes no liability for this information and the product described herein. These are averages and are not guaranteed as conditions of sale. -

Kelp Cultivation Effectively Improves Water Quality and Regulates Phytoplankton Community in a Turbid, Highly Eutrophic Bay
Science of the Total Environment 707 (2020) 135561 Contents lists available at ScienceDirect Science of the Total Environment journal homepage: www.elsevier.com/locate/scitotenv Kelp cultivation effectively improves water quality and regulates phytoplankton community in a turbid, highly eutrophic bay Zhibing Jiang a,b,d, Jingjing Liu a,ShangluLic,YueChena,PingDua,b,YuanliZhua,YiboLiaoa,d, Quanzhen Chen a, Lu Shou a, Xiaojun Yan e, Jiangning Zeng a,⁎, Jianfang Chen a,d a Key Laboratory of Marine Ecosystem and Biogeochemistry, State Oceanic Administration & Second Institute of Oceanography, Ministry of Natural Resources, Hangzhou, China b Function Laboratory for Marine Fisheries Science and Food Production Processes, Pilot National Laboratory for Marine Science and Technology (Qingdao), Qingdao, China c Marine Monitoring and Forecasting Center of Zhejiang Province, Hangzhou, China d State Key Laboratory of Satellite Ocean Environment Dynamics, Second Institute of Oceanography, Ministry of Natural Resources, Hangzhou, China e Key Laboratory of Applied Marine Biotechnology, Ministry of Education, Marine College of Ningbo University, Ningbo, China HIGHLIGHTS GRAPHICAL ABSTRACT • Kelp farming alleviated eutrophication and acidification. • Kelp farming greatly relieved light limi- tation and increased phytoplankton bio- mass. • Kelp farming appreciably enhanced phytoplankton diversity. • Kelp farming reduced the dominance of dinoflagellate Prorocentrum minimum. • Phytoplankton community differed sig- nificantly between the kelp farm and control area. article info abstract Article history: Coastal eutrophication and its associated harmful algal blooms have emerged as one of the most severe environ- Received 19 September 2019 mental problems worldwide. Seaweed cultivation has been widely encouraged to control eutrophication and Received in revised form 11 November 2019 algal blooms. Among them, cultivated kelp (Saccharina japonica) dominates primarily by production and area. -

WMSDB - Worldwide Mollusc Species Data Base
WMSDB - Worldwide Mollusc Species Data Base Family: TURBINIDAE Author: Claudio Galli - [email protected] (updated 07/set/2015) Class: GASTROPODA --- Clade: VETIGASTROPODA-TROCHOIDEA ------ Family: TURBINIDAE Rafinesque, 1815 (Sea) - Alphabetic order - when first name is in bold the species has images Taxa=681, Genus=26, Subgenus=17, Species=203, Subspecies=23, Synonyms=411, Images=168 abyssorum , Bolma henica abyssorum M.M. Schepman, 1908 aculeata , Guildfordia aculeata S. Kosuge, 1979 aculeatus , Turbo aculeatus T. Allan, 1818 - syn of: Epitonium muricatum (A. Risso, 1826) acutangulus, Turbo acutangulus C. Linnaeus, 1758 acutus , Turbo acutus E. Donovan, 1804 - syn of: Turbonilla acuta (E. Donovan, 1804) aegyptius , Turbo aegyptius J.F. Gmelin, 1791 - syn of: Rubritrochus declivis (P. Forsskål in C. Niebuhr, 1775) aereus , Turbo aereus J. Adams, 1797 - syn of: Rissoa parva (E.M. Da Costa, 1778) aethiops , Turbo aethiops J.F. Gmelin, 1791 - syn of: Diloma aethiops (J.F. Gmelin, 1791) agonistes , Turbo agonistes W.H. Dall & W.H. Ochsner, 1928 - syn of: Turbo scitulus (W.H. Dall, 1919) albidus , Turbo albidus F. Kanmacher, 1798 - syn of: Graphis albida (F. Kanmacher, 1798) albocinctus , Turbo albocinctus J.H.F. Link, 1807 - syn of: Littorina saxatilis (A.G. Olivi, 1792) albofasciatus , Turbo albofasciatus L. Bozzetti, 1994 albofasciatus , Marmarostoma albofasciatus L. Bozzetti, 1994 - syn of: Turbo albofasciatus L. Bozzetti, 1994 albulus , Turbo albulus O. Fabricius, 1780 - syn of: Menestho albula (O. Fabricius, 1780) albus , Turbo albus J. Adams, 1797 - syn of: Rissoa parva (E.M. Da Costa, 1778) albus, Turbo albus T. Pennant, 1777 amabilis , Turbo amabilis H. Ozaki, 1954 - syn of: Bolma guttata (A. Adams, 1863) americanum , Lithopoma americanum (J.F. -

Phylum MOLLUSCA
285 MOLLUSCA: SOLENOGASTRES-POLYPLACOPHORA Phylum MOLLUSCA Class SOLENOGASTRES Family Lepidomeniidae NEMATOMENIA BANYULENSIS (Pruvot, 1891, p. 715, as Dondersia) Occasionally on Lafoea dumosa (R.A.T., S.P., E.J.A.): at 4 positions S.W. of Eddystone, 42-49 fm., on Lafoea dumosa (Crawshay, 1912, p. 368): Eddystone, 29 fm., 1920 (R.W.): 7, 3, 1 and 1 in 4 hauls N.E. of Eddystone, 1948 (V.F.) Breeding: gonads ripe in Aug. (R.A.T.) Family Neomeniidae NEOMENIA CARINATA Tullberg, 1875, p. 1 One specimen Rame-Eddystone Grounds, 29.12.49 (V.F.) Family Proneomeniidae PRONEOMENIA AGLAOPHENIAE Kovalevsky and Marion [Pruvot, 1891, p. 720] Common on Thecocarpus myriophyllum, generally coiled around the base of the stem of the hydroid (S.P., E.J.A.): at 4 positions S.W. of Eddystone, 43-49 fm. (Crawshay, 1912, p. 367): S. of Rame Head, 27 fm., 1920 (R.W.): N. of Eddystone, 29.3.33 (A.J.S.) Class POLYPLACOPHORA (=LORICATA) Family Lepidopleuridae LEPIDOPLEURUS ASELLUS (Gmelin) [Forbes and Hanley, 1849, II, p. 407, as Chiton; Matthews, 1953, p. 246] Abundant, 15-30 fm., especially on muddy gravel (S.P.): at 9 positions S.W. of Eddystone, 40-43 fm. (Crawshay, 1912, p. 368, as Craspedochilus onyx) SALCOMBE. Common in dredge material (Allen and Todd, 1900, p. 210) LEPIDOPLEURUS, CANCELLATUS (Sowerby) [Forbes and Hanley, 1849, II, p. 410, as Chiton; Matthews. 1953, p. 246] Wembury West Reef, three specimens at E.L.W.S.T. by J. Brady, 28.3.56 (G.M.S.) Family Lepidochitonidae TONICELLA RUBRA (L.) [Forbes and Hanley, 1849, II, p. -

Optimization of Seedling Production Using Vegetative Gametophytes Of
Optimization of seedling production using vegetative gametophytes of Alaria esculenta Aires Duarte Mestrado em Biologia Funcional e Biotecnologia de Plantas Departamento de Biologia 2017 Orientador Isabel Sousa Pinto, associate professor, CIIMAR Coorientador Jorunn Skjermo, Senior Scientist, SINTEF OCEAN 2 3 Acknowledgments First and foremost, I would like to express my sincere gratitude to: professor Isabel Sousa Pinto of Universidade do Porto and senior research scientist Jorunn Skjermo of SINTEF ocean. From the beginning I had an interest to work aboard with macroalgae, after talking with prof. Isabel Sousa Pinto about this interest, she immediately suggested me a few places that I could look over. One of the suggestions was SINTEF ocean where I got to know Jorunn Skjermo. The door to Jorunn’s office was always open whenever I ran into a trouble spot or had a question about my research. She consistently allowed this study to be my own work, but steered me in the right the direction whenever she thought I needed it. Thank you!! I want to thank Isabel Azevedo, Silje Forbord and Kristine Steinhovden for all the guidance provided in the beginning and until the end of my internship. I would also like to thank the experts who were involved in the different subjects of my research project: Arne Malzahn, Torfinn Solvang-Garten, Trond Storseth and to the amazing team of SINTEF ocean. I also want to thank my master’s director professor Paula Melo, who was a relentless person from the first day, always taking care of her “little F1 plants”. A huge thanks to my fellows Mónica Costa, Fernando Pagels and Leonor Martins for all the days and nights that we spent working and studying hard. -

Medicinal Values of Seaweeds
Medicinal Values of Seaweeds Authors Abdul Kader Mohiuddin Assistant Professor, Department of Pharmacy, World University, Dhanmondi, Dhaka, Bangladesh Publication Month and Year: November 2019 Pages: 69 E-BOOK ISBN: 978-81-943354-3-6 Academic Publications C-11, 169, Sector-3, Rohini, Delhi Website: www.publishbookonline.com Email: [email protected] Phone: +91-9999744933 Page | 1 Page | 2 Medicinal Values of Seaweeds Abstract The global economic effect of the five driving chronic diseases- malignancy, diabetes, psychological instability, CVD, and respiratory disease- could reach $47 trillion throughout the following 20 years, as indicated by an examination by the World Economic Forum (WEF). As per the WHO, 80% of the total people principally those of developing countries depend on plant- inferred medicines for social insurance. The indicated efficacies of seaweed inferred phytochemicals are demonstrating incredible potential in obesity, T2DM, metabolic syndrome, CVD, IBD, sexual dysfunction and a few cancers. Hence, WHO, UN-FAO, UNICEF and governments have indicated a developing enthusiasm for these offbeat nourishments with wellbeing advancing impacts. Edible marine macro-algae (seaweed) are of intrigue in view of their incentive in nutrition and medicine. Seaweeds contain a few bioactive substances like polysaccharides, proteins, lipids, polyphenols, and pigments, all of which may have useful wellbeing properties. People devour seaweed as nourishment in different structures: crude as salad and vegetable, pickle with sauce or with vinegar, relish or improved jams and furthermore cooked for vegetable soup. By cultivating seaweed, coastal people are getting an alternative livelihood just as propelling their lives. In 2005, world seaweed generation totaled 14.7 million tons which has dramatically increased (30.4 million tons) in 2015. -

Neoproterozoic Origin and Multiple Transitions to Macroscopic Growth in Green Seaweeds
Neoproterozoic origin and multiple transitions to macroscopic growth in green seaweeds Andrea Del Cortonaa,b,c,d,1, Christopher J. Jacksone, François Bucchinib,c, Michiel Van Belb,c, Sofie D’hondta, f g h i,j,k e Pavel Skaloud , Charles F. Delwiche , Andrew H. Knoll , John A. Raven , Heroen Verbruggen , Klaas Vandepoeleb,c,d,1,2, Olivier De Clercka,1,2, and Frederik Leliaerta,l,1,2 aDepartment of Biology, Phycology Research Group, Ghent University, 9000 Ghent, Belgium; bDepartment of Plant Biotechnology and Bioinformatics, Ghent University, 9052 Zwijnaarde, Belgium; cVlaams Instituut voor Biotechnologie Center for Plant Systems Biology, 9052 Zwijnaarde, Belgium; dBioinformatics Institute Ghent, Ghent University, 9052 Zwijnaarde, Belgium; eSchool of Biosciences, University of Melbourne, Melbourne, VIC 3010, Australia; fDepartment of Botany, Faculty of Science, Charles University, CZ-12800 Prague 2, Czech Republic; gDepartment of Cell Biology and Molecular Genetics, University of Maryland, College Park, MD 20742; hDepartment of Organismic and Evolutionary Biology, Harvard University, Cambridge, MA 02138; iDivision of Plant Sciences, University of Dundee at the James Hutton Institute, Dundee DD2 5DA, United Kingdom; jSchool of Biological Sciences, University of Western Australia, WA 6009, Australia; kClimate Change Cluster, University of Technology, Ultimo, NSW 2006, Australia; and lMeise Botanic Garden, 1860 Meise, Belgium Edited by Pamela S. Soltis, University of Florida, Gainesville, FL, and approved December 13, 2019 (received for review June 11, 2019) The Neoproterozoic Era records the transition from a largely clear interpretation of how many times and when green seaweeds bacterial to a predominantly eukaryotic phototrophic world, creat- emerged from unicellular ancestors (8). ing the foundation for the complex benthic ecosystems that have There is general consensus that an early split in the evolution sustained Metazoa from the Ediacaran Period onward. -

Xoimi AMERICAN COXCIIOLOGY
S31ITnS0NIAN MISCEllANEOUS COLLECTIOXS. BIBLIOGIIAPHY XOimi AMERICAN COXCIIOLOGY TREVIOUS TO THE YEAR 18G0. PREPARED FOR THE SMITHSONIAN INSTITUTION BY . W. G. BINNEY. PART II. FOKEIGN AUTHORS. WASHINGTON: SMITHSONIAN INSTITUTION. JUNE, 1864. : ADYERTISEMENT, The first part of the Bibliography of American Conchology, prepared for the Smithsonian Institution by Mr. Binuey, was published in March, 1863, and embraced the references to de- scriptions of shells by American authors. The second part of the same work is herewith presented to the public, and relates to species of North American shells referred to by European authors. In foreign works binomial authors alone have been quoted, and no species mentioned which is not referred to North America or some specified locality of it. The third part (in an advanced stage of preparation) will in- clude the General Index of Authors, the Index of Generic and Specific names, and a History of American Conchology, together with any additional references belonging to Part I and II, that may be met with. JOSEPH HENRY, Secretary S. I. Washington, June, 1864. (" ) PHILADELPHIA COLLINS, PRINTER. CO]^TENTS. Advertisement ii 4 PART II.—FOREIGN AUTHORS. Titles of Works and Articles published by Foreign Authors . 1 Appendix II to Part I, Section A 271 Appendix III to Part I, Section C 281 287 Appendix IV .......... • Index of Authors in Part II 295 Errata ' 306 (iii ) PART II. FOEEIGN AUTHORS. ( V ) BIBLIOGRxVPHY NOETH AMERICAN CONCHOLOGY. PART II. Pllipps.—A Voyage towards the North Pole, &c. : by CON- STANTiNE John Phipps. Loudou, ITTJc. Pa. BIBLIOGRAPHY OF [part II. FaliricillS.—Fauna Grcenlandica—systematice sistens ani- malia GrcEulandite occidentalis liactenus iudagata, &c., secun dum proprias observatioues Othonis Fabricii. -

Edible Seaweed from Wikipedia, the Free Encyclopedia
Edible seaweed From Wikipedia, the free encyclopedia Edible seaweed are algae that can be eaten and used in the preparation of food. They typically contain high amounts of fiber.[1] They may belong to one of several groups of multicellular algae: the red algae, green algae, and brown algae. Seaweeds are also harvested or cultivated for the extraction of alginate, agar and carrageenan, gelatinous substances collectively known as hydrocolloids or phycocolloids. Hydrocolloids have attained commercial significance, especially in food production as food A dish of pickled spicy seaweed additives.[2] The food industry exploits the gelling, water-retention, emulsifying and other physical properties of these hydrocolloids. Most edible seaweeds are marine algae whereas most freshwater algae are toxic. Some marine algae contain acids that irritate the digestion canal, while some others can have a laxative and electrolyte-balancing effect.[3] The dish often served in western Chinese restaurants as 'Crispy Seaweed' is not seaweed but cabbage that has been dried and then fried.[4] Contents 1 Distribution 2 Nutrition and uses 3 Common edible seaweeds 3.1 Red algae (Rhodophyta) 3.2 Green algae 3.3 Brown algae (Phaeophyceae) 3.3.1 Kelp (Laminariales) 3.3.2 Fucales 3.3.3 Ectocarpales 4 See also 5 References 6 External links Distribution Seaweeds are used extensively as food in coastal cuisines around the world. Seaweed has been a part of diets in China, Japan, and Korea since prehistoric times.[5] Seaweed is also consumed in many traditional European societies, in Iceland and western Norway, the Atlantic coast of France, northern and western Ireland, Wales and some coastal parts of South West England,[6] as well as Nova Scotia and Newfoundland. -

The Giant Sea Mammal That Went Extinct in Less Than Three Decades
The Giant Sea Mammal That Went Extinct in Less Than Three Decades The quick disappearance of the 30-foot animal helped to usher in the modern science of human-caused extinctions. JACOB MIKANOWSKI, THE ATLANTIC 4/19/17 HTTPS://WWW.THEATLANTIC.COM/SCIENCE/ARCHIVE/2017/04/PLEIST OSEACOW/522831/ The Pleistocene, the geologic era immediately preceding our own, was an age of giants. North America was home to mastodons and saber-tooth cats; mammoths and wooly rhinos roamed Eurasia; giant lizards and bear-sized wombats strode across the Australian outback. Most of these giants died at the by the end of the last Ice Age, some 14,000 years ago. Whether this wave of extinctions was caused by climate change, overhunting by humans, or some combination of both remains a subject of intense debate among scientists. Complicating the picture, though, is the fact that a few Pleistocene giants survived the Quaternary extinction event and nearly made it intact to the present. Most of these survivor species found refuge on islands. Giant sloths were still living on Cuba 6,000 years ago, long after their relatives on the mainland had died out. The last wooly mammoths died out just 4,000 years ago. They lived in a small herd on Wrangel Island north of the Bering Strait between the Chukchi and East Siberian Seas. Two-thousand years ago, gorilla-sized lemurs were still living on Madagascar. A thousand years ago, 12-foot-tall moa birds were still foraging in the forests of New Zealand. Unlike the other long-lived megafauna, Steller’s sea cows, one of the last of the Pleistocene survivors to die out, found their refuge in a remote scrape of the ocean instead of on land. -

Download PDF Version
MarLIN Marine Information Network Information on the species and habitats around the coasts and sea of the British Isles Sea lace or Dead man's rope (Chorda filum) MarLIN – Marine Life Information Network Biology and Sensitivity Key Information Review Nicola White 2006-11-07 A report from: The Marine Life Information Network, Marine Biological Association of the United Kingdom. Please note. This MarESA report is a dated version of the online review. Please refer to the website for the most up-to-date version [https://www.marlin.ac.uk/species/detail/1366]. All terms and the MarESA methodology are outlined on the website (https://www.marlin.ac.uk) This review can be cited as: White, N. 2006. Chorda filum Sea lace or Dead man's rope. In Tyler-Walters H. and Hiscock K. (eds) Marine Life Information Network: Biology and Sensitivity Key Information Reviews, [on-line]. Plymouth: Marine Biological Association of the United Kingdom. DOI https://dx.doi.org/10.17031/marlinsp.1366.1 The information (TEXT ONLY) provided by the Marine Life Information Network (MarLIN) is licensed under a Creative Commons Attribution-Non-Commercial-Share Alike 2.0 UK: England & Wales License. Note that images and other media featured on this page are each governed by their own terms and conditions and they may or may not be available for reuse. Permissions beyond the scope of this license are available here. Based on a work at www.marlin.ac.uk (page left blank) Date: 2006-11-07 Sea lace or Dead man's rope (Chorda filum) - Marine Life Information Network See online review for distribution map Chorda filum.