Associated Heterotrophic Protists, Stereomyxa Ramosa, Nitzschia Alba and Labyrinthula Sp
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
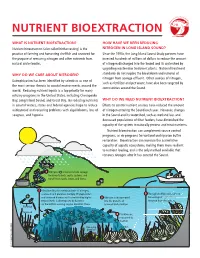
Nutrient Bioextraction L
soun nd d s sla tu i d g y n o NUTRIENT BIOEXTRACTION l WHAT IS NUTRIENT BIOEXTRACTION? HOW HAVE WE BEEN REDUCING Nutrient bioextraction (also called bioharvesting) is the NITROGEN IN LONG ISLAND SOUND? practice of farming and harvesting shellfish and seaweed for Since the 1990s, the Long Island Sound Study partners have the purpose of removing nitrogen and other nutrients from invested hundreds of millions of dollars to reduce the amount natural water bodies. of nitrogen discharged into the Sound and its watershed by upgrading wastewater treatment plants. National treatment WHY DO WE CARE ABOUT NITROGEN? standards do not require the breakdown and removal of nitrogen from sewage effluent. Other sources of nitrogen, Eutrophication has been identified by scientists as one of such as fertilizer and pet waste, have also been targeted by the most serious threats to coastal environments around the communities around the Sound. world. Reducing nutrient inputs is a top priority for many estuary programs in the United States, including Chesapeake Bay, Long Island Sound, and Great Bay. By reducing nutrients WHY DO WE NEED NUTRIENT BIOEXTRACTION? in coastal waters, states and federal agencies hope to reduce Efforts to control nutrient sources have reduced the amount widespread and recurring problems with algal blooms, loss of of nitrogen entering the Sound each year. However, changes seagrass, and hypoxia. in the Sound and its watershed, such as wetland loss and decreased populations of filter feeders, have diminished the capacity of the system to naturally process and treat nutrients. Nutrient bioextraction can complement source control programs, as do programs for wetland and riparian buffer restoration. -

Physiology and Biochemistry of the Tropical Seagrass Thalassia Testudinum in Response to Hypersalinity Stress and Labyrinthula Sp
UNF Digital Commons UNF Graduate Theses and Dissertations Student Scholarship 2011 Physiology and Biochemistry of the Tropical Seagrass Thalassia testudinum in Response to Hypersalinity Stress and Labyrinthula sp. Infection Stacey Marie Trevathan-Tackett University of North Florida Suggested Citation Trevathan-Tackett, Stacey Marie, "Physiology and Biochemistry of the Tropical Seagrass Thalassia testudinum in Response to Hypersalinity Stress and Labyrinthula sp. Infection" (2011). UNF Graduate Theses and Dissertations. 391. https://digitalcommons.unf.edu/etd/391 This Master's Thesis is brought to you for free and open access by the Student Scholarship at UNF Digital Commons. It has been accepted for inclusion in UNF Graduate Theses and Dissertations by an authorized administrator of UNF Digital Commons. For more information, please contact Digital Projects. © 2011 All Rights Reserved Physiology and Biochemistry of the Tropical Seagrass Thalassia testudinum in Response to Hypersalinity Stress and Labyrinthula sp. Infection by Stacey Marie Trevathan-Tackett A thesis submitted to the Department of Biology in partial fulfillment of the requirements for the degree of Master of Science in Biology University of North Florida College of Arts and Sciences December, 2011 Unpublished Work © 2011 Stacey Marie Trevathan-Tackett Signature Deleted Signature Deleted Signature Deleted Signature Deleted Signature Deleted Signature Deleted Acknowledgements I first would like to acknowledge NOAA and the Nancy Foster Scholarship for funding both school and living expenses for the majority of my Master‟s career allowing me to focus all of my time and efforts toward research. Also, I would like to thank the Dodson Grant, the Coastal Biology program and the Graduate School at UNF for their continued financial support and resources during graduate school. -

Kelp Cultivation Effectively Improves Water Quality and Regulates Phytoplankton Community in a Turbid, Highly Eutrophic Bay
Science of the Total Environment 707 (2020) 135561 Contents lists available at ScienceDirect Science of the Total Environment journal homepage: www.elsevier.com/locate/scitotenv Kelp cultivation effectively improves water quality and regulates phytoplankton community in a turbid, highly eutrophic bay Zhibing Jiang a,b,d, Jingjing Liu a,ShangluLic,YueChena,PingDua,b,YuanliZhua,YiboLiaoa,d, Quanzhen Chen a, Lu Shou a, Xiaojun Yan e, Jiangning Zeng a,⁎, Jianfang Chen a,d a Key Laboratory of Marine Ecosystem and Biogeochemistry, State Oceanic Administration & Second Institute of Oceanography, Ministry of Natural Resources, Hangzhou, China b Function Laboratory for Marine Fisheries Science and Food Production Processes, Pilot National Laboratory for Marine Science and Technology (Qingdao), Qingdao, China c Marine Monitoring and Forecasting Center of Zhejiang Province, Hangzhou, China d State Key Laboratory of Satellite Ocean Environment Dynamics, Second Institute of Oceanography, Ministry of Natural Resources, Hangzhou, China e Key Laboratory of Applied Marine Biotechnology, Ministry of Education, Marine College of Ningbo University, Ningbo, China HIGHLIGHTS GRAPHICAL ABSTRACT • Kelp farming alleviated eutrophication and acidification. • Kelp farming greatly relieved light limi- tation and increased phytoplankton bio- mass. • Kelp farming appreciably enhanced phytoplankton diversity. • Kelp farming reduced the dominance of dinoflagellate Prorocentrum minimum. • Phytoplankton community differed sig- nificantly between the kelp farm and control area. article info abstract Article history: Coastal eutrophication and its associated harmful algal blooms have emerged as one of the most severe environ- Received 19 September 2019 mental problems worldwide. Seaweed cultivation has been widely encouraged to control eutrophication and Received in revised form 11 November 2019 algal blooms. Among them, cultivated kelp (Saccharina japonica) dominates primarily by production and area. -

Genetic Diversity of Labyrinthula Spp. in Seagrass Zostera Marina Beds in the North Atlantic Ocean and the Baltic Sea
Genetic diversity of Labyrinthula spp. in seagrass Zostera marina beds in the North Atlantic Ocean and the Baltic Sea Submitted by Matsapume Detcharoen In partial fulfillment of the requirements for the Degree of Master of Science in Biology Ludwig-Maximilians-Universität München Faculty of Biology August 2015 Abstract Die sogenannte Seegras – „Wasting Disease“ führte in den 1930er Jahren zu einem Massenrückgang des Seegrases Zostera marina im nördlichen Atlantik. Labyrinthula zosterae ist der potentielle Erreger der Krankheit, der zu dem Verlust von Seegrasbetten führen kann. Dieser Organismus infiziert die Blätter des Seegrases. Frühere Studien, die die Vielfalt der Labyrinthula spp untersuchten, basierten auf der Kultivierung von Labyrinthula. Diese Studie ist die erste Studie, die Blattproben zur molekularen Identifizierung von Labyrinthula verwendet und erforscht die Vielfalt der Gattung Labyrinthula. Proben von sechs Standorten wurden untersucht, dabei wurde ein 290 bp lange Teilsequenz der 18S rDNA kloniert und Sanger sequenziert. Das Abgleichen der erhaltenen Sequenzen mit NCBI GenBank mittels des blast Algorithmus ergab, dass die meisten Sequenzen von 77 bis 100% mit L. zosterae übereinstimmen, während inige Sequenzen der Standorte Wackerballig und Sandspollen eine 84-94 prozentige Übereinstimmung mit Labyrinthula spp zeigten. Keine der mutmaßlichen Labyrinthula spp. Sequenzen wurden mit bekannten Sequenzen aus der Datenbank zusammengefasst. Es wurden sieben OTUs generiert. Der Test von verschiedenen DNA-Extraktionmethoden ergab für ein Pflanzen- und ein Gewebe-Extraktionskit ähnliche Ergebnisse. Nichtsdestotrotz hat das Pflanzen-Kit mehr Vorteile wegen geringerer Kosten und dem geringeren Zeitaufwand. Zusammenfassend zeigt diese Studie, dass bisher unbekannte Labyrinthula spp. in den Seegraspopulationen vorkommen, und dass das in dieser Studie verwendete Verfahren für weitere Anwendungen geeignet ist. -

Neoproterozoic Origin and Multiple Transitions to Macroscopic Growth in Green Seaweeds
Neoproterozoic origin and multiple transitions to macroscopic growth in green seaweeds Andrea Del Cortonaa,b,c,d,1, Christopher J. Jacksone, François Bucchinib,c, Michiel Van Belb,c, Sofie D’hondta, f g h i,j,k e Pavel Skaloud , Charles F. Delwiche , Andrew H. Knoll , John A. Raven , Heroen Verbruggen , Klaas Vandepoeleb,c,d,1,2, Olivier De Clercka,1,2, and Frederik Leliaerta,l,1,2 aDepartment of Biology, Phycology Research Group, Ghent University, 9000 Ghent, Belgium; bDepartment of Plant Biotechnology and Bioinformatics, Ghent University, 9052 Zwijnaarde, Belgium; cVlaams Instituut voor Biotechnologie Center for Plant Systems Biology, 9052 Zwijnaarde, Belgium; dBioinformatics Institute Ghent, Ghent University, 9052 Zwijnaarde, Belgium; eSchool of Biosciences, University of Melbourne, Melbourne, VIC 3010, Australia; fDepartment of Botany, Faculty of Science, Charles University, CZ-12800 Prague 2, Czech Republic; gDepartment of Cell Biology and Molecular Genetics, University of Maryland, College Park, MD 20742; hDepartment of Organismic and Evolutionary Biology, Harvard University, Cambridge, MA 02138; iDivision of Plant Sciences, University of Dundee at the James Hutton Institute, Dundee DD2 5DA, United Kingdom; jSchool of Biological Sciences, University of Western Australia, WA 6009, Australia; kClimate Change Cluster, University of Technology, Ultimo, NSW 2006, Australia; and lMeise Botanic Garden, 1860 Meise, Belgium Edited by Pamela S. Soltis, University of Florida, Gainesville, FL, and approved December 13, 2019 (received for review June 11, 2019) The Neoproterozoic Era records the transition from a largely clear interpretation of how many times and when green seaweeds bacterial to a predominantly eukaryotic phototrophic world, creat- emerged from unicellular ancestors (8). ing the foundation for the complex benthic ecosystems that have There is general consensus that an early split in the evolution sustained Metazoa from the Ediacaran Period onward. -

Edible Seaweed from Wikipedia, the Free Encyclopedia
Edible seaweed From Wikipedia, the free encyclopedia Edible seaweed are algae that can be eaten and used in the preparation of food. They typically contain high amounts of fiber.[1] They may belong to one of several groups of multicellular algae: the red algae, green algae, and brown algae. Seaweeds are also harvested or cultivated for the extraction of alginate, agar and carrageenan, gelatinous substances collectively known as hydrocolloids or phycocolloids. Hydrocolloids have attained commercial significance, especially in food production as food A dish of pickled spicy seaweed additives.[2] The food industry exploits the gelling, water-retention, emulsifying and other physical properties of these hydrocolloids. Most edible seaweeds are marine algae whereas most freshwater algae are toxic. Some marine algae contain acids that irritate the digestion canal, while some others can have a laxative and electrolyte-balancing effect.[3] The dish often served in western Chinese restaurants as 'Crispy Seaweed' is not seaweed but cabbage that has been dried and then fried.[4] Contents 1 Distribution 2 Nutrition and uses 3 Common edible seaweeds 3.1 Red algae (Rhodophyta) 3.2 Green algae 3.3 Brown algae (Phaeophyceae) 3.3.1 Kelp (Laminariales) 3.3.2 Fucales 3.3.3 Ectocarpales 4 See also 5 References 6 External links Distribution Seaweeds are used extensively as food in coastal cuisines around the world. Seaweed has been a part of diets in China, Japan, and Korea since prehistoric times.[5] Seaweed is also consumed in many traditional European societies, in Iceland and western Norway, the Atlantic coast of France, northern and western Ireland, Wales and some coastal parts of South West England,[6] as well as Nova Scotia and Newfoundland. -

Kelp Aquaculture
Aquaculture in Shared Waters Kelp Aquaculture Sarah Redmond1 ; Samuel Belknap2 ; Rebecca Clark Uchenna3 “Kelp” are large brown marine macroalgae species native to New England and traditionally wild harvested for food. There are three commercially important kelp species in Maine—sugar kelp (Saccharina latissima), winged kelp (Alaria esculenta), and horsetail kelp (Laminaria digitata). Maine is developing techniques for culturing kelp on sea farms as a way for fishermen and farmers to diversify their operations while providing a unique, high quality, nutritious vegetable seafood for new and existing markets. Kelp is grown on submerged horizontal long lines on leased sea farms from September to May, making it a “winter crop” for Maine. The simple farm design, winter season, and relatively low startup costs allow for new and existing sea farmers to experiment with this newly developing type of aquaculture on Maine’s coast. “Kelp” can refer to sugar kelp (Saccharina latissima), Alaria (Alaria esculenta), or horsetail kelp (Laminaria digitata). Sugar kelp has been cultivated in Maine for several years, and successful experimental cultivation has been done with species such as Alaria. These photos are examples of the cultivation stages of sugar kelp. Microscopic Seeded kelp line Kelp line at time of kelp seed harvest 1 Sarah Redmond • Marine Extension Associate, Maine Sea Grant College Program and University of Maine Cooperative Extension 33 Salmon Farm Road Franklin, ME • 207.422.6289 • [email protected] 2 Samuel Belknap • University of Maine • 234C South Stevens Hall Orono, ME • 207.992.7726 • [email protected] 3 Rebecca Clark Uchenna • Island Institute • Rockland, ME • 207.691.2505 • [email protected] Is there a viable market for Q: kelps grown in Maine? aine is home to a handful of consumers are looking for healthier industry, the existing producers and Mcompanies that harvest sea alternatives. -

Controlled Sampling of Ribosomally Active Protistan Diversity in Sediment-Surface Layers Identifies Putative Players in the Marine Carbon Sink
The ISME Journal (2020) 14:984–998 https://doi.org/10.1038/s41396-019-0581-y ARTICLE Controlled sampling of ribosomally active protistan diversity in sediment-surface layers identifies putative players in the marine carbon sink 1,2 1 1 3 3 Raquel Rodríguez-Martínez ● Guy Leonard ● David S. Milner ● Sebastian Sudek ● Mike Conway ● 1 1 4,5 6 7 Karen Moore ● Theresa Hudson ● Frédéric Mahé ● Patrick J. Keeling ● Alyson E. Santoro ● 3,8 1,9 Alexandra Z. Worden ● Thomas A. Richards Received: 6 October 2019 / Revised: 4 December 2019 / Accepted: 17 December 2019 / Published online: 9 January 2020 © The Author(s) 2020. This article is published with open access Abstract Marine sediments are one of the largest carbon reservoir on Earth, yet the microbial communities, especially the eukaryotes, that drive these ecosystems are poorly characterised. Here, we report implementation of a sampling system that enables injection of reagents into sediments at depth, allowing for preservation of RNA in situ. Using the RNA templates recovered, we investigate the ‘ribosomally active’ eukaryotic diversity present in sediments close to the water/sediment interface. We 1234567890();,: 1234567890();,: demonstrate that in situ preservation leads to recovery of a significantly altered community profile. Using SSU rRNA amplicon sequencing, we investigated the community structure in these environments, demonstrating a wide diversity and high relative abundance of stramenopiles and alveolates, specifically: Bacillariophyta (diatoms), labyrinthulomycetes and ciliates. The identification of abundant diatom rRNA molecules is consistent with microscopy-based studies, but demonstrates that these algae can also be exported to the sediment as active cells as opposed to dead forms. -

The Classification of Lower Organisms
The Classification of Lower Organisms Ernst Hkinrich Haickei, in 1874 From Rolschc (1906). By permission of Macrae Smith Company. C f3 The Classification of LOWER ORGANISMS By HERBERT FAULKNER COPELAND \ PACIFIC ^.,^,kfi^..^ BOOKS PALO ALTO, CALIFORNIA Copyright 1956 by Herbert F. Copeland Library of Congress Catalog Card Number 56-7944 Published by PACIFIC BOOKS Palo Alto, California Printed and bound in the United States of America CONTENTS Chapter Page I. Introduction 1 II. An Essay on Nomenclature 6 III. Kingdom Mychota 12 Phylum Archezoa 17 Class 1. Schizophyta 18 Order 1. Schizosporea 18 Order 2. Actinomycetalea 24 Order 3. Caulobacterialea 25 Class 2. Myxoschizomycetes 27 Order 1. Myxobactralea 27 Order 2. Spirochaetalea 28 Class 3. Archiplastidea 29 Order 1. Rhodobacteria 31 Order 2. Sphaerotilalea 33 Order 3. Coccogonea 33 Order 4. Gloiophycea 33 IV. Kingdom Protoctista 37 V. Phylum Rhodophyta 40 Class 1. Bangialea 41 Order Bangiacea 41 Class 2. Heterocarpea 44 Order 1. Cryptospermea 47 Order 2. Sphaerococcoidea 47 Order 3. Gelidialea 49 Order 4. Furccllariea 50 Order 5. Coeloblastea 51 Order 6. Floridea 51 VI. Phylum Phaeophyta 53 Class 1. Heterokonta 55 Order 1. Ochromonadalea 57 Order 2. Silicoflagellata 61 Order 3. Vaucheriacea 63 Order 4. Choanoflagellata 67 Order 5. Hyphochytrialea 69 Class 2. Bacillariacea 69 Order 1. Disciformia 73 Order 2. Diatomea 74 Class 3. Oomycetes 76 Order 1. Saprolegnina 77 Order 2. Peronosporina 80 Order 3. Lagenidialea 81 Class 4. Melanophycea 82 Order 1 . Phaeozoosporea 86 Order 2. Sphacelarialea 86 Order 3. Dictyotea 86 Order 4. Sporochnoidea 87 V ly Chapter Page Orders. Cutlerialea 88 Order 6. -

An Assessment of the Influence of Host Species, Age, and Thallus Part
diversity Article An Assessment of the Influence of Host Species, Age, and Thallus Part on Kelp-Associated Diatoms Ntambwe Albert Serge Mayombo 1,* , Roksana Majewska 2,3 and Albertus J. Smit 1,4,* 1 Department of Biodiversity and Conservation Biology, University of the Western Cape, Bellville 7535, South Africa 2 Unit for Environmental Sciences and Management, School of Biological Sciences, North-West University, Potchefstroom 2520, South Africa; [email protected] 3 South African Institute for Aquatic Biodiversity (SAIAB), Grahamstown 6140, South Africa 4 Elwandle Coastal Node, South African Environmental Observation Network (SAEON), Port Elizabeth 6013, South Africa * Correspondence: [email protected] (N.A.S.M.); [email protected] (A.J.S.) Received: 26 June 2020; Accepted: 17 August 2020; Published: 8 October 2020 Abstract: Diatom community composition and abundances on different thallus parts of adult and juvenile specimens of Ecklonia maxima and Laminaria pallida were examined in False Bay, South Africa, using light and scanning electron microscopy. Altogether, 288 thallus portions were analysed. 2 Diatom abundances ranged from 0 to 404 cells mm− and were generally higher on E. maxima and juvenile thalli than L. pallida and adult specimens. Moreover, diatom abundances differed between the various thallus parts, being highest on the upper blade and lowest on the primary blade. A total of 48 diatom taxa belonging to 28 genera were found. Gomphoseptatum Medlin, Nagumoea Witkowski and Kociolek, Cocconeis Ehrenberg, and Navicula Bory were the most frequently occurring genera, being present in 84%, 65%, 62.5%, and 45% of the analysed samples, respectively. Among these, Cocconeis and Gomphoseptatum were the most abundant, contributing 50% and 27% of total diatom cells counted collectively across all samples. -

Seaweed Seaweeds Are an Important Food and Medicine to Humans Everywhere That They Grow
Seaweed Seaweeds are an important food and medicine to humans everywhere that they grow. They have been harvested by Salish People off the Pacific coast for countless generations and are used for thickening soups, seasoning foods, and for baking foods in cooking pits. Seaweeds are exceptionally high in minerals, trace elements and protein. They can be preserved through careful drying in the sun or near a fire. Where they grow: In salt water at middle to low tidal zones. Each type of seaweed has a tidal zone habitat – from sea lettuce and bladder wrack that grow on rocks in upper tidal zones to bull whip kelp, which grows in deep waters. Season: Like other edible plant greens, seaweeds are harvested in spring and early summer when they are most vital. In late summer and fall they get tougher and begin to deteriorate. How to Harvest: It is very important to harvest seaweeds from clean waters because they can absorb environmental toxins. The safest places are open waters of the Pacific with strong current flow away from cities, towns or industrial runoff. Washington State allows us to harvest 10 pounds wet weight per day on public beaches and you need a shellfish/seaweed license to harvest. The bottom of the seaweed or “hold-fast” anchors on to rocks while leaves grow upward toward the light like an undersea forest. Make sure you leave the holdfast and at least a quarter of the seaweed plant so it can grow back. Do not clear-cut any area so that the seaweed can continue to thrive. -

Short Communication on the Classification of The
SHORT COMMUNICATION ON THE CLASSIFICATION OF THE GENERA Labyrinthula, Schizochytrium AND Thraustochytrium (Labyrinthulids AND Thraustochytrids) Øjvind Moestrup Biological Institute, University of Copenhagen Universitetsparken 4 , DK- 2100 Copenhagen, Denmark E-mail: [email protected] Citation as: Moestrup Øjvind, 2019. On the classification of the genera Labyrinthula, Schizochytrium and Thraustochytrium (Labyrinthulids and Thraustochytrids). Tap chi Sinh hoc, 41(2): xx–xx. https://doi.org/10.15625/0866-7160/v41n2.xxxxx Species of the genera Labyrinthula, Schizochytrium, Thraustochytrium and related organisms have recently attracted attention in biotechnology, and here is a short note on how to classify these rather special organisms. The labyrinthulids and thraustochytrids belong to the heterokonts, a large group of very diverse organisms, from microscopic unicells to metre-long brown algae. The heterokonts comprise species that were formerly classified as algae, fungi and/or protozoa. Many heterokonts are autotrophic and contain chloroplasts, and such organisms are often classified as algae (golden algae, brown algae). Labyrinthulids and thraustochytrids, however, are heterotrophic and lack chloroplasts. They were until recently known as Labyrinthulomycetes or Labyrinthulea , indeed the new classification of Adl et al. (2019) uses the first of these names. It is an unfortunate name as it gives the misleading impression that they are fungi. Honigberg et al. (1964) and others considered them protozoa. Are heterokonts algae, protozoa or fungi? And, more specifically, what are labyrinthulids and thraustochytrids? The heterokonts are thought to be a very old group (probably precambrian), and this may account for their huge morphological diversity. In the WORMS list (World Register of Marine Species) heterokonts are classified as the Infrakingdom Heterokonta .