Stabilizing Selection and the Structural Variability of Flowers Within Species
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

List of Vascular Plants Endemic to Britain, Ireland and the Channel Islands 2020
British & Irish Botany 2(3): 169-189, 2020 List of vascular plants endemic to Britain, Ireland and the Channel Islands 2020 Timothy C.G. Rich Cardiff, U.K. Corresponding author: Tim Rich: [email protected] This pdf constitutes the Version of Record published on 31st August 2020 Abstract A list of 804 plants endemic to Britain, Ireland and the Channel Islands is broken down by country. There are 659 taxa endemic to Britain, 20 to Ireland and three to the Channel Islands. There are 25 endemic sexual species and 26 sexual subspecies, the remainder are mostly critical apomictic taxa. Fifteen endemics (2%) are certainly or probably extinct in the wild. Keywords: England; Northern Ireland; Republic of Ireland; Scotland; Wales. Introduction This note provides a list of vascular plants endemic to Britain, Ireland and the Channel Islands, updating the lists in Rich et al. (1999), Dines (2008), Stroh et al. (2014) and Wyse Jackson et al. (2016). The list includes endemics of subspecific rank or above, but excludes infraspecific taxa of lower rank and hybrids (for the latter, see Stace et al., 2015). There are, of course, different taxonomic views on some of the taxa included. Nomenclature, taxonomic rank and endemic status follows Stace (2019), except for Hieracium (Sell & Murrell, 2006; McCosh & Rich, 2018), Ranunculus auricomus group (A. C. Leslie in Sell & Murrell, 2018), Rubus (Edees & Newton, 1988; Newton & Randall, 2004; Kurtto & Weber, 2009; Kurtto et al. 2010, and recent papers), Taraxacum (Dudman & Richards, 1997; Kirschner & Štepànek, 1998 and recent papers) and Ulmus (Sell & Murrell, 2018). Ulmus is included with some reservations, as many taxa are largely vegetative clones which may occasionally reproduce sexually and hence may not merit species status (cf. -

An Introgression Analysis of Quantitative Trait Loci That Contribute to a Morphological Difference Between Drosophila Simulans and D
Copyight 0 1997 by the Genetics Societv of America An Introgression Analysis of Quantitative Trait Loci That Contribute to a Morphological Difference Between Drosophila simulans and D. mauritiana Cathy C. Laurie, John R. True, Jianjun Liu and John M. Mercer Department of Zoology, Duke University, Durham, North Carolina 27708 Manuscript received August 12, 1996 Accepted for publication October 29, 1996 ABSTRACT Drosophila simulans and D. maum'tiana differ markedly in morphology of the posterior lobe, a male- specific genitalic structure. Bothsize and shapeof the lobe can be quantifiedby a morphometric variable, PC1, derived from principal components and Fourier analyses. The genetic architectureof the species difference in PC1 was investigated previously by composite interval mapping, which revealed largely additive inheritance, with a minimum of eight quantitative trait loci (QTL) affecting the trait. This analysis was extended by introgression of marked segments of the maun'tiana third chromosome into a simulans background by repeated backcrossing. Thetwo types of experiment are consistentin suggesting that several QTL on the third chromosome may have effects in the range of 10-15% of the parental difference andthat all or nearly all QTL have effects in the same direction. Since the parental difference is large (30.4 environmental standard deviations), effects of this magnitude can produce alternative homozygotes with little overlap in phenotype. However, these estimates may not reflect the effects of individual loci, since each interval or introgressed segment may contain multiple QTL. The consistent direction of allelic effects suggests a history of directional selection on the posterior lobe. HE genetic analysis of a quantitative trait usually ual genes that affect continuously variable traits. -

Condition of Designated Sites
Scottish Natural Heritage Condition of Designated Sites Contents Chapter Page Summary ii Condition of Designated Sites (Progress to March 2010) Site Condition Monitoring 1 Purpose of SCM 1 Sites covered by SCM 1 How is SCM implemented? 2 Assessment of condition 2 Activities and management measures in place 3 Summary results of the first cycle of SCM 3 Action taken following a finding of unfavourable status in the assessment 3 Natural features in Unfavourable condition – Scottish Government Targets 4 The 2010 Condition Target Achievement 4 Amphibians and Reptiles 6 Birds 10 Freshwater Fauna 18 Invertebrates 24 Mammals 30 Non-vascular Plants 36 Vascular Plants 42 Marine Habitats 48 Coastal 54 Machair 60 Fen, Marsh and Swamp 66 Lowland Grassland 72 Lowland Heath 78 Lowland Raised Bog 82 Standing Waters 86 Rivers and Streams 92 Woodlands 96 Upland Bogs 102 Upland Fen, Marsh and Swamp 106 Upland Grassland 112 Upland Heathland 118 Upland Inland Rock 124 Montane Habitats 128 Earth Science 134 www.snh.gov.uk i Scottish Natural Heritage Summary Background Scotland has a rich and important diversity of biological and geological features. Many of these species populations, habitats or earth science features are nationally and/ or internationally important and there is a series of nature conservation designations at national (Sites of Special Scientific Interest (SSSI)), European (Special Area of Conservation (SAC) and Special Protection Area (SPA)) and international (Ramsar) levels which seek to protect the best examples. There are a total of 1881 designated sites in Scotland, although their boundaries sometimes overlap, which host a total of 5437 designated natural features. -

Fall 2013 NARGS
Rock Garden uar terly � Fall 2013 NARGS to ADVERtISE IN thE QuARtERly CoNtACt [email protected] Let me know what yo think A recent issue of a chapter newsletter had an item entitled “News from NARGS”. There were comments on various issues related to the new NARGS website, not all complimentary, and then it turned to the Quarterly online and raised some points about which I would be very pleased to have your views. “The good news is that all the Quarterlies are online and can easily be dowloaded. The older issues are easy to read except for some rather pale type but this may be the result of scanning. There is amazing information in these older issues. The last three years of the Quarterly are also online but you must be a member to read them. These last issues are on Allen Press’s BrightCopy and I find them harder to read than a pdf file. Also the last issue of the Quarterly has 60 extra pages only available online. Personally I find this objectionable as I prefer all my content in a printed bulletin.” This raises two points: Readability of BrightCopy issues versus PDF issues Do you find the BrightCopy issues as good as the PDF issues? Inclusion of extra material in online editions only. Do you object to having extra material in the online edition which can not be included in the printed edition? Please take a moment to email me with your views Malcolm McGregor <[email protected]> CONTRIBUTORS All illustrations are by the authors of articles unless otherwise stated. -

Microevolution and the Genetics of Populations Microevolution Refers to Varieties Within a Given Type
Chapter 8: Evolution Lesson 8.3: Microevolution and the Genetics of Populations Microevolution refers to varieties within a given type. Change happens within a group, but the descendant is clearly of the same type as the ancestor. This might better be called variation, or adaptation, but the changes are "horizontal" in effect, not "vertical." Such changes might be accomplished by "natural selection," in which a trait within the present variety is selected as the best for a given set of conditions, or accomplished by "artificial selection," such as when dog breeders produce a new breed of dog. Lesson Objectives ● Distinguish what is microevolution and how it affects changes in populations. ● Define gene pool, and explain how to calculate allele frequencies. ● State the Hardy-Weinberg theorem ● Identify the five forces of evolution. Vocabulary ● adaptive radiation ● gene pool ● migration ● allele frequency ● genetic drift ● mutation ● artificial selection ● Hardy-Weinberg theorem ● natural selection ● directional selection ● macroevolution ● population genetics ● disruptive selection ● microevolution ● stabilizing selection ● gene flow Introduction Darwin knew that heritable variations are needed for evolution to occur. However, he knew nothing about Mendel’s laws of genetics. Mendel’s laws were rediscovered in the early 1900s. Only then could scientists fully understand the process of evolution. Microevolution is how individual traits within a population change over time. In order for a population to change, some things must be assumed to be true. In other words, there must be some sort of process happening that causes microevolution. The five ways alleles within a population change over time are natural selection, migration (gene flow), mating, mutations, or genetic drift. -

Savory Guide
The Herb Society of America's Essential Guide to Savory 2015 Herb of the Year 1 Introduction As with previous publications of The Herb Society of America's Essential Guides we have developed The Herb Society of America's Essential The Herb Society Guide to Savory in order to promote the knowledge, of America is use, and delight of herbs - the Society's mission. We hope that this guide will be a starting point for studies dedicated to the of savory and that you will develop an understanding and appreciation of what we, the editors, deem to be an knowledge, use underutilized herb in our modern times. and delight of In starting to put this guide together we first had to ask ourselves what it would cover. Unlike dill, herbs through horseradish, or rosemary, savory is not one distinct species. It is a general term that covers mainly the educational genus Satureja, but as time and botanists have fractured the many plants that have been called programs, savories, the title now refers to multiple genera. As research and some of the most important savories still belong to the genus Satureja our main focus will be on those plants, sharing the but we will also include some of their close cousins. The more the merrier! experience of its Savories are very historical plants and have long been utilized in their native regions of southern members with the Europe, western Asia, and parts of North America. It community. is our hope that all members of The Herb Society of America who don't already grow and use savories will grow at least one of them in the year 2015 and try cooking with it. -

Patterns and Power of Phenotypic Selection in Nature
Articles Patterns and Power of Phenotypic Selection in Nature JOEL G. KINGSOLVER AND DAVID W. PFENNIG Phenotypic selection occurs when individuals with certain characteristics produce more surviving offspring than individuals with other characteristics. Although selection is regarded as the chief engine of evolutionary change, scientists have only recently begun to measure its action in the wild. These studies raise numerous questions: How strong is selection, and do different types of traits experience different patterns of selection? Is selection on traits that affect mating success as strong as selection on traits that affect survival? Does selection tend to favor larger body size, and, if so, what are its consequences? We explore these questions and discuss the pitfalls and future prospects of measuring selection in natural populations. Keywords: adaptive landscape, Cope’s rule, natural selection, rapid evolution, sexual selection henotypic selection occurs when individuals with selection on traits that affect survival stronger than on those Pdifferent characteristics (i.e., different phenotypes) that affect only mating success? In this article, we explore these differ in their survival, fecundity, or mating success. The idea and other questions about the patterns and power of phe- of phenotypic selection traces back to Darwin and Wallace notypic selection in nature. (1858), and selection is widely accepted as the primary cause of adaptive evolution within natural populations.Yet Darwin What is selection, and how does it work? never attempted to measure selection in nature, and in the Selection is the nonrandom differential survival or repro- century following the publication of On the Origin of Species duction of phenotypically different individuals. -

ISSN: 2320-5407 Int. J. Adv. Res. 5(7), 1301-1312
ISSN: 2320-5407 Int. J. Adv. Res. 5(7), 1301-1312 Journal Homepage: - www.journalijar.com Article DOI: 10.21474/IJAR01/4841 DOI URL: http://dx.doi.org/10.21474/IJAR01/4841 RESEARCH ARTICLE FLORA OF CHEPAN MOUNTAIN (WESTERN BULGARIA). Dimcho Zahariev. Faculty of Natural Sciences, Department of Plant Protection, Botany and Zoology, University of Shumen, Bulgaria. …………………………………………………………………………………………………….... Manuscript Info Abstract ……………………. ……………………………………………………………… Manuscript History Chepan Mountain is located in Western Bulgaria. It is part of Balkan Mountains on the territory of Balkan Peninsula in Southern Europe. As Received: 13 May 2017 a result of this study in Chepan Mountain on the territory of only 25 Final Accepted: 15 June 2017 km2 were found 784 species of wild vascular plants from 378 genera Published: July 2017 and 84 families. Such amazing biodiversity can be found in Southern Europe only. The floristic analysis indicates that the most of the Key words:- families and the genera are represented by a small number of inferior Chepan Mountain, floristic analysis, taxa. The hemicryptophytes dominate among the life forms with vascular plants 53.32%. The biological types are represented mainly by perennial herbaceous plants (59.57%). In the flora of the Mountain there are 49 floristic elements. The most of the species are European-Asiatic floristic elements (14.54%), followed by European-Mediterranean floristic elements (13.78%) and subMediterranean floristic elements (13.52%). Among the vascular plants, there are 26 Balkan endemic species, 4 Bulgarian endemic species and 26 relic species. The species with protection statute are 66 species. The anthropophytes among the vascular plants are 390 species (49.74%). -

Pollinating Flies (Diptera): a Major Contribution to Plant Diversity and Agricultural Production Axel Ssymank', C.A
Pollinating Flies (Diptera): A major contribution to plant diversity and agricultural production Axel Ssymank', C.A. Kearns2, Thomas Pape 3 andF. Christian Thompson 4 Abstract. Diptera are one of the three largest and most diverse animal groups in the world. As an often neglected but important group of pollinators, they play a significant role in agrobiodiversity and the biodiversity of plants everywhere. Flies are present in almost all habitats and biomes and for many medicinal, food and ornamental plants, pollinating flies guarantee or enhance seed and fruit production. They are important in the natural landscape, in agriculture and in greenhouses, and have recently come into use in the production of seeds for seed banks. The Sao Paulo Pollinator Initiative, the CBD, and Pollinator secretariats were important starting points in the international recognition of pollinator importance. However, large gaps in our knowledge of the role of Diptera in pollination networks need to be addressed in order to sustain agriculture and to enable appropriate responses to climate change. At this 9th Conference of the Parties we would like to draw attention to the role of often-neglected Dipteran pollinators, to stress their current importance and potential future, use as pollinators in agriculture. A case study on flower flies that act as important pollinators, as adults, and major biocontrol agents, as larvae, illustrates their double importance for agriculture. Keywords. Diptera, Agrobiodiversity, Pollination, Flower Flies, Bio-Control, medicinal plants, ornamental plants AUTHORS' ADDRESSES: 2004) were presented. These initial programs focused ' Corresponding author: Axel Ssymank c/o Federal Agency for Nature on the pollination services of bees. -

Scottish Biodiversity Strategy Post-2020: a Statement of Intent
Scottish Biodiversity Strategy Post-2020: A Statement of Intent December 2020 INTRODUCTION have to change how we interact with and care for nature. The world faces the challenges of climate change and biodiversity loss. Globally, The twin global crises of biodiversity loss nationally and locally an enormous effort and climate change require us to work is needed to tackle these closely linked with nature to secure a healthier planet. issues. As we move from the United Our Climate Change Plan update outlines Nations Decade on Biodiversity to the new, boosted and accelerated policies, beginning of the United Nations Decade putting us on a pathway to our ambitious of Ecosystem Restoration, with climate change targets and to deliver a preparations being made for the range of co-benefits including for Convention on Biological Diversity’s biodiversity. The way we use land and Conference of the Parties 15 to be held in sea has to simultaneously enable the 2021, this is an appropriate time to reflect transition to net zero as part of a green and set out our broad intentions on how economic recovery, adapt to a changing we will approach the development of a climate and improve the state of nature. new post-2020 Scottish Biodiversity This is an unprecedented tripartite Strategy. challenge. The new UN Decade signals the massive The devastating impact of COVID-19 has effort needed and it is highlighted our need to be far more resilient to pandemics and other ‘shocks’ “…a rallying call for the protection which may arise from degraded nature. and revival of ecosystems around Our Programme for Government and the world, for the benefit of people Climate Change Plan update set out and nature… Only with healthy steps we will take to support a green ecosystems can we enhance recovery. -
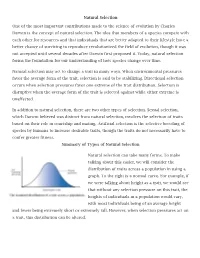
Natural Selection One of the Most Important Contributions Made to The
Natural Selection One of the most important contributions made to the science of evolution by Charles Darwin is the concept of natural selection. The idea that members of a species compete with each other for resources and that individuals that are better adapted to their lifestyle have a better chance of surviving to reproduce revolutionized the field of evolution, though it was not accepted until several decades after Darwin first proposed it. Today, natural selection forms the foundation for our understanding of how species change over time. Natural selection may act to change a trait in many ways. When environmental pressures favor the average form of the trait, selection is said to be stabilizing. Directional selection occurs when selection pressures favor one extreme of the trait distribution. Selection is disruptive when the average form of the trait is selected against while either extreme is unaffected. In addition to natural selection, there are two other types of selection. Sexual selection, which Darwin believed was distinct from natural selection, involves the selection of traits based on their role in courtship and mating. Artificial selection is the selective breeding of species by humans to increase desirable traits, though the traits do not necessarily have to confer greater fitness. Summary of Types of Natural Selection Natural selection can take many forms. To make talking about this easier, we will consider the distribution of traits across a population in using a graph. To the right is a normal curve. For example, if we were talking about height as a trait, we would see that without any selection pressure on this trait, the heights of individuals in a population would vary, with most individuals being of an average height and fewer being extremely short or extremely tall. -

Questa Pianta, Molto Piccola, Con I Fiori Viola Intenso
16 luglio 2010 (f.f.) questa pianta, molto piccola, con i fiori viola intenso può non attrarre l’attenzione dell’escursionista distratto, ma una volta che l’ha vista è inevitabile che si fermi ad ammirarla e fotografarla. IL GENERE ACINOS Famiglia Lamiaceae (o Labiatae) Acinos L. fu classificato da Linneo nel 1753. Il nome generico Acinos è preso dal latino ăcĭnǒs (usato da Plinio per riferirsi a una qualità di basilico o a erba aromatica in generale) a sua volta derivato dal greco ακιυος. Il termine greco si riferiva alla pianta di timo e, per la somiglianza tra i due generi, Linneo chiamò l’Acinos alpinus con il nome Tymus alpinus. Il genere Acinos comprende una decina di specie legnose annuali o perenni, ma di corta vita, originarie del sud-Europa e dell’Asia occidentale. Hanno dimensioni ridotte non raggiungendo mezzo metro di altezza. ACINOS ALPINUS Acinos alpinus (L.) Moench subsp. alpinus Classificata da Moench1 nel 1794 Conosciuta anche come: Thymus alpinus L., Satureja alpina (L.) Scheele, Calamintha alpina (L.) Lam. Conosciuta volgarmente come: acino alpino, santoreggia alpina. Il nome specifico alpinus deriva dal latino alpīnus, a, um (= delle Alpi) a indicare i luoghi in cui la pianta prospera. Così riporta il botanico apuano Pietro Pellegrini2: 1150. – Calamintha alpina – Lam. [Acinos alpinus (L.) Moench subsp. alpinus] 1 Conrad Moench (1744-1805) fu un botanico tedesco. Fu professore all’università austriaca di Marburg. 2 Pietro Pellegrini “Flora della Provincia di Apuania ossia Rassegna delle piante fanerogame indigene, inselvatichite, avventizie esotiche e di quelle largamente coltivate nel territorio di Apuania e delle crittogame vascolari e cellulari, con la indicazione dei luoghi di raccolta”, Stab.