© Copyright 2019 Rochelle M. Kelly
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

State Park Contact Sheet Last Updated November 2016
WASHINGTON STATE PARKS AND RECREATION COMMISSION Film Permit Application State Park Contact Sheet Last Updated November 2016 AREA MANAGER PHONE PARK NAME PARK AREA ADDRESS EMAIL (@parks.wa.gov) REGION Sharon Soelter ALTA LAKE STATE PARK (509) 923-2473 Alta Lake State Park Alta Lake Area 1B OTTO ROAD [email protected] Eastern PATEROS WA 98846 Brian Hageman FORT WORDEN STATE PARK Anderson Lake (360) 344-4442 Olympic View Area 200 BATTERY WAY State Park [email protected] Southwest PORT TOWNSEND, WA 98368-3621 Chris Guidotti BATTLE GROUND STATE PARK Battle Ground Lake (360) 687-4621 Battle Ground Area PO BOX 148 State Park [email protected] Southwest HEISSON, WA 98622 Kevin Kratochvil RASAR STATE PARK (360) 757-0227 Bay View State Park Rasar Area 38730 CAPE HORN ROAD [email protected] Northwest CONCRETE, WA 98237 Chris Guidotti BATTLE GROUND STATE PARK Beacon Rock (509) 427-8265 Battle Ground Area PO BOX 148 State Park [email protected] Southwest HEISSON, WA 98622 Joel Pillers BELFAIR STATE PARK (360) 275-0668 Belfair State Park South Sound Area 3151 N.E. SR 300 [email protected] Southwest BELFAIR, WA 98528 Jack Hartt DECEPTION PASS STATE PARK Ben Ure Island Marine (360) 675-3767 Deception Pass Area 41020 STATE ROUTE 20 State Park [email protected] Northwest OAK HARBOR, WA 98277 Ted Morris BIRCH BAY STATE PARK (360) 371-2800 Birch Bay State Park Birch Bay Area 5105 HELWEG ROAD [email protected] Northwest BLAINE WA 98230 Dave Roe MANCHESTER STATE PARK Blake Island Marine (360) 731-8330 Blake -

Education Research Experience
Rochelle Kelly Curriculum Vitae 472 Life Sciences Building Ph.D. Candidate University of Washington [email protected] Seattle WA, 98195 EDUCATION 2013- 2018 Ph.D. Candidate, Department of Biology, University of Washington, Seattle, WA Dissertation Title: Diversity, distributions, and ecology of bats among the San Juan Islands. Advisor: Dr. Sharlene Santana 2013 B.S. Conservation & Resource Studies, High Honors, University of California, Berkeley, CA Honors thesis: Influence of landscape heterogeneity on bat activity in vineyards. Advisor: Dr. Erica Bree Rosenblum 2007 A.S. Veterinary Technology, Western Career College, Pleasant Hill, CA RESEARCH EXPERIENCE 2018 Genetic and morphological diversity of Myotis spp. in Washington State. Collected morphometric and genetic data to compare patterns morphological and genetic diversity among two Pacific Northwest Myotis spp. (M. lucifugus and M. yumanensis) across Washington State. Department of Biology and the Burke Museum, University of Washington, Seattle, WA. Advisor: Dr. Sharlene Santana. 2013-2018 Diversity, distributions, and ecology of bats among the San Juan Islands. Carried out field and lab work to collect and analyze tissue, guano, & distribution data from bats among the San Juan Islands to study diversity and distribution patterns. Department of Biology, University of Washington, Seattle, WA. Advisor: Dr. Sharlene Santana. 2014-2018 Comparison of morphological and molecular identification of Washington’s Myotis spp. Designed and managed course project for BIOL/FISH 340, where students carried out morphological and molecular identification of Myotis spp. that were submitted to the Washington Department of Health for Rabies testing. Department of Biology and the Burke Museum, University of Washington, Seattle, WA. Advisor: Dr. Sharlene Santana and Dr. -

Lime Kiln Point State Park (San Juan Island)
Ranald MacDonald’s Grave Your guide to state parks in the Auto-accessible parks Auto-accessible parks Lime Kiln Point State Park Moran State Park Spencer Spit State Park Obstruction Pass State Park Welcome (San Juan Island) (Orcas Island) (Lopez Island) (Orcas Island) San Juan The San Juan archipelago north of Puget At Lime Kiln Point State Park, the loud neighbors Pass through the welcome arch at Moran State Spencer Spit on Lopez Island provides dramatic Obstruction Pass State Park is one of the few Sound is like no other place on earth. The cluster gear up for a party that runs from spring into Park, and time begins to slow. You’ll find yourself views of Decatur and Blakely islands and Mount public beaches on famed Orcas Island. of 400 islands and rocks in the Salish Sea is a fall. Those would be the spouting Orcas, fin- in a Northwest island frame of mind, free to relax, Constitution on Orcas Island, and it features a Though most people flock to its bigger world unto itself. It is a world where people are slapping gray whales, barking sea lions and breathe and head into the vast, varied terrain. rare sand spit enclosed by a salt-chuck lagoon. neighbor, Moran State Park, this property’s friendly and hearty, where the land smells like splashing porpoises. Hike, cycle or drive to the summit of Mount The effect is a driftwood-scattered beach on quiet beauty is unsurpassed. Clear waters lap at the sea, and wind, art and history are celebrated. Constitution for expansive views of the San Juan one side of the spit and a spongy marsh on the pebbly beaches, and madrone trees cling to Obstruction Pass Islands For island dwellers and visitors, the pace of life other. -

Migratory Bird
-o a o — z -n c: ST! <g»S z Ra 1 »•£ = =H H> - 2 » 2 "> -< r- cu o =: Z ,—. National Wildlife Refuges of s The "Migratory Bird" Refuges -n DO "_- ""= = „ = = S=> zs: oo = c« J> -n o, « —I -o ac to s> SS; i— 3» Dungeness National Wildlife 2 m o)2 2 a H Puget Sound <= a. — c/3 m Refuge oo a. m -TO OO a, _ DO and What Are Migratory Birds? Why Do They Come Here? Dungeness Spit is formed by eroding soil, wind, and water C3 CD j3£ currents, and stretches for five and one-half miles along the = m While some birds live in one area year-round, "migratory Like people, migratory birds need food, water and shelter. Strait of Juan de Fuca. It breaks the rough sea waves to <=> Coastal Washington birds" make regular seasonal flights from one area to Both Nisqually and Dungeness National Wildlife Refuges form a quiet bay, sand and gravel beaches, and tideflats -n another. These birds usually fly between wintering areas and have abundant supplies of water and offer a smorgasbord of —I where wildlife can find food and protection from wind, m summer breeding grounds. Although many species go north foods to suit a variety of avian tastes. Shorebirds may feast on waves, and pounding surf. The bay and estuary of the and south, others travel between coastal breeding areas and mudflat invertebrates while goldfinches prefer the fluffy Dungeness River produce micro-organisms that form the —I the open sea. Migration allows them to escape the short days m seeds of thistles and dandelions. -

A Model for Measuring the Benefits of State Parks for the Washington State Parks And
6 A Model for Measuring the Benefits of State Parks for the Washington State Parks and january 201 january Recreation Commission Prepared By: Prepared For: Earth Economics Washington State Parks and Tacoma, Washington Recreation Commission Olympia, Washington Primary Authors: Tania Briceno, PhD, Ecological Economist, Earth Economics Johnny Mojica, Research Analyst, Earth Economics Suggested Citation: Briceno, T., Mojica, J. 2016. Statewide Land Acquisition and New Park Development Strategy. Earth Economics, Tacoma, WA. Acknowledgements: Thanks to all who supported this project including the Earth Economics team: Greg Schundler (GIS analysis), Corrine Armistead (Research, Analysis, and GIS), Jessica Hanson (editor), Josh Reyneveld (managing director), Sage McElroy (design); the Washington State Parks and Recreation Commission: Tom Oliva, Katie Manning, Steve Hahn, Steve Brand, Nikki Fields, Peter Herzog and others. We would also like to thank our Board of Directors for their continued guidance and support: Ingrid Rasch, David Cosman, Sherry Richardson, David Batker, and Joshua Farley. The authors are responsible for the content of this report. Cover image: Washington State Department of Transportation ©2016 by Earth Economics. Reproduction of this publication for educational or other non-commercial purposes is authorized without prior written permission from the copyright holder provided the source is fully acknowledged. Reproduction of this publication for resale or other commercial purposes is prohibited without prior written permission of the copyright holder. Executive Summary Washington’s state parks provide a myriad of benefits to both urban and rural environments and nearby residents. Green spaces within state parks provide direct benefits to the populations living in close proximity. For example, the forests within state parks provide outdoor recreational opportunities, and they also help to store water and control flooding during heavy rainfalls, improve air quality, and regulate the local climate. -

Mammalogy Newsletter 3.30.16
2016 Mammalogy Collection Newsletter SHARLENE E. SANTANA – CURATOR JEFFREY E. BRADLEY – COLLECTION MANAGER A GREAT YEAR FOR MAMMALOGY Greetings from the Burke Museum’s Mammalogy Collection! We are excited to provide you with the first installment of our annual newsletter, which highlights some of our major achievements in 2015. We had a busy and productive year, as we continued to fulfill the collection’s mission to archive mammal diversity, support scientific inquiry, and contribute to science teaching and public outreach within Washington State and beyond. 2015 Activity Summary The collection continued its steady growth in 2015, thanks to fieldwork Specimens catalogued 697 efforts by Mammalogy staff, institutional partnerships, and chance events, Research visits 387 as detailed in several sections within this newsletter. The collection Research projects at the Burke 27 continued to receive specimens from large scale research initiatives in Specimens examined at the Burke 2,419 Washington State, including the Elwha River Dam Removal and the Mt. St. Specimens loaned 426 Helens Biodiversity Monitoring Project. Scientific publications 18 Students served 420 Visitors engaged in public outreach 1,600+ Mammalogy specimens were used in a wide variety of research projects, spanning the fields of biology, engineering, archeology, paleontology and art. Previous research at Burke Mammalogy yielded 18 scientific publications in 2015 (full list on page 6). Eleven classes, serving 420 students at the University of Washington, the Northwest School, Alderleaf Wilderness College, and Seattle Public Schools used specimens from the collection to provide hands-on learning experiences. Last November, over 1,000 visitors came through the Burke’s door for the 10th annual Meet the Mammals family day. -

San Juan County Community Wildfire Protection Plan Steering Committee
San Juan County, Washington Community Wildfire Protection Plan/Wildfire Risk Assessment 2010 Satellite Island Fire Adopted by the San Juan County Council August 2012 This plan was developed by the San Juan County Community Wildfire Protection Plan steering committee. Acknowledgements This Community Wildfire Protection Plan represents the efforts and cooperation of a number of organizations and agencies working together to improve preparedness for wildfire events while reducing factors of risk. Waldron Island Fire Brigade 2012 - /Wildfire Risk Assessment /Wildfire Community Wildfire Protection Wildfire Plan Community Washington Washington To obtain copies of this plan contact: County, San Juan County Emergency Management PO Box 669 Friday Harbor, WA 98250 360-370-7612 [email protected] San Juan Table of Contents ACKNOWLEDGEMENTS ............................................................................................................................................................ II FOREWORD .................................................................................................................................................................................... 1 CHAPTER 1 ..................................................................................................................................................................................... 3 OVERVIEW OF THIS PLAN AND ITS DEVELOPMENT ....................................................................................................... 3 GOALS AND GUIDING PRINCIPLES -

Do Roost-Excavating Bats Have Stronger Skulls?
Biological Journal of the Linnean Society, 2011, 102, 1–10. With 3 figures Do roost-excavating bats have stronger skulls? SHARLENE E. SANTANA1* and ELIZABETH R. DUMONT2 1Graduate Program in Organismic and Evolutionary Biology, University of Massachusetts Amherst, Amherst, MA, USA 2Department of Biology, University of Massachusetts Amherst, Amherst, MA, USA Received 14 April 2010; accepted for publication 27 July 2010bij_1551 1..10 In addition to feeding, many vertebrates use their skulls for other functions that are highly relevant to fitness. One such function is roost excavation by the bat Lophostoma silvicolum. Males of this species use their canines to create cavities inside active termite nests, which are significantly harder than the prey they eat. Here we investigate whether the skull of L. silvicolum is specialized for roost excavation relative to the ecologically similar species Tonatia saurophila and Micronycteris hirsuta, which do not excavate roosts. We conducted a finite element analysis that simulated roost excavating and feeding behaviours. These analyses were informed by our observations of feeding and roost-excavating behaviours, bite force, and dissections of the cranial musculature of the three bat species. During the simulation of roost excavation (bilateral canine biting), our data indicate that most regions of the skull of L. silvicolum exhibit less stress than those of T. saurophila and M. hirsuta; however, the latter exhibited the lowest peak stress at the zygomatic arches. During loads that simulate feeding (bilateral molar biting), the three species exhibit similar stress levels. It is not clear whether L. silvicolum has a skull shape that is stronger under the loads imposed by excavation, but it does exhibit relatively higher bilateral canine bite forces that are generated via relatively larger temporalis muscles. -

25 JUL 2021 Index Aaron Creek 17385 179 Aaron Island
26 SEP 2021 Index 401 Angoon 17339 �� � � � � � � � � � 287 Baranof Island 17320 � � � � � � � 307 Anguilla Bay 17404 �� � � � � � � � 212 Barbara Rock 17431 � � � � � � � 192 Index Anguilla Island 17404 �� � � � � � � 212 Bare Island 17316 � � � � � � � � 296 Animal and Plant Health Inspection Ser- Bar Harbor 17430 � � � � � � � � 134 vice � � � � � � � � � � � � 24 Barlow Cove 17316 �� � � � � � � � 272 Animas Island 17406 � � � � � � � 208 Barlow Islands 17316 �� � � � � � � 272 A Anita Bay 17382 � � � � � � � � � 179 Barlow Point 17316 � � � � � � � � 272 Anita Point 17382 � � � � � � � � 179 Barnacle Rock 17401 � � � � � � � 172 Aaron Creek 17385 �� � � � � � � � 179 Annette Bay 17428 � � � � � � � � 160 Barnes Lake 17382 �� � � � � � � � 172 Aaron Island 17316 �� � � � � � � � 273 Annette Island 17434 � � � � � � � 157 Baron Island 17420 �� � � � � � � � 122 Aats Bay 17402� � � � � � � � � � 277 Annette Point 17434 � � � � � � � 156 Bar Point Basin 17430� � � � � � � 134 Aats Point 17402 �� � � � � � � � � 277 Annex Creek Power Station 17315 �� � 263 Barren Island 17434 � � � � � � � 122 Abbess Island 17405 � � � � � � � 203 Appleton Cove 17338 � � � � � � � 332 Barren Island Light 17434 �� � � � � 122 Abraham Islands 17382 � � � � � � 171 Approach Point 17426 � � � � � � � 162 Barrie Island 17360 � � � � � � � � 230 Abrejo Rocks 17406 � � � � � � � � 208 Aranzazu Point 17420 � � � � � � � 122 Barrier Islands 17386, 17387 �� � � � 228 Adams Anchorage 17316 � � � � � � 272 Arboles Islet 17406 �� � � � � � � � 207 Barrier Islands 17433 -

How Evolution Made the Monkey Face
DATA The Matrix HOWHOW EVOLUTIONEVOLUTION MMAADEDE TTHHEE MONKEYMONKEY FFAACECE The complexity, or lack thereof, on a simian’s face reveals a great deal about its society. curious thing happens to white-faced capuchin monkeys Santana found that the complexity of a species’ facial color when they anoint their bodies with mud and plant mat- patterns is tightly linked to certain social systems. Species that ter, a natural insect repellent: With their heads and faces live in larger groups tend to have plainer faces than those living in Aslathered in goop, these highly social primates lose their ability to smaller groups. Primates in large social groups most likely benefit recognize each other. Previously friendly monkeys can become from plain faces that allow for a greater range of expressions, she fighting foes. This abrupt change in behavior hints at the impor- explains. But among primates in smaller groups (and those that tance of facial expressions for recognition, University of Wash- live near many different species, regardless of group size), more ington evolutionary biologist Sharlene Santana says, and could complex patterning makes it easier to recognize members of their help to explain why primate faces are so wildly divergent: Some own species, so they can focus their mating efforts and territorial species, like white-faced capuchins, have monotone hair and skin disputes where it counts. color; others, like the northern owl monkey, sport a dramatic mix The connection between group size and facial evolution tells us of fur and flesh tones. something about our own ancient history too, since human faces Biologists have long seen primates’ facial expressions during are about as plain as it gets. -

Curriculum Vitae, August 2013 2
Elizabeth R. Dumont Department of Biology University of Massachusetts 221 Morrill Science Center Amherst, MA 01003-9297 Tel: 413 545-3565 Fax: 413 545-3243 E-mail: [email protected] Lab web page: www.biomesh.org Education Ph.D. 1993 S.U.N.Y. at Stony Brook, Anthropology M.A. 1989 S.U.N.Y. at Stony Brook, Anthropology B.A. 1985 Indiana University (Bloomington), Anthropology (with honors) Academic Positions 2009 - present Professor, Department of Biology, University of Massachusetts, Amherst 2006 - 2009 Associate Professor, Department of Biology, University of Massachusetts, Amherst 2001 - 2006 Assistant Professor, Department of Biology, University of Massachusetts, Amherst 1996 - 2001 Assistant Professor, Department of Anatomy, Northeastern Ohio Universities College of Medicine 1996 - present Research Associate, Section of Mammals, Carnegie Museum of Natural History 1993 - 1996 Postdoctoral Teaching Fellow, Department of Neurobiology, University of Pittsburgh, School of Medicine Grant Support 2014-2019 National Science Foundation, Collaborative Research, “Discovering genomic and developmental mechanisms that underlie sensory innovations critical to adaptive diversification.” (P.I.) Direct Costs: $294,092, Indirect costs: $158,736 2014-2017 National Science Foundation, Collaborative Research, “Strain in cartilaginous fish skeletons” (P.I.) Direct Costs: $ 139,371, Indirect Costs: $ 82,229 2010-2012 National Science Foundation, Division of Undergraduate Education, “Seeing the Forest and the Trees” (P.I.) Direct Costs: $135,217, Indirect Costs: $64,774 2008 - 2012 National Science Foundation, Division of Biological Databases and Bioinformatics, “Biomesh: A digital resource collection on the biology- engineering interface” (P.I.) Direct Costs: $670,425 (includes $7,500 REU supplement) Indirect Costs: $320,913 2006 Research Leadership Award. -
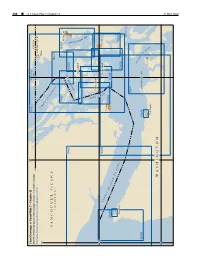
CPB7 C12 WEB.Pdf
488 ¢ U.S. Coast Pilot 7, Chapter 12 Chapter 7, Pilot Coast U.S. 124° 123° Chart Coverage in Coast Pilot 7—Chapter 12 18421 BOUNDARY NOAA’s Online Interactive Chart Catalog has complete chart coverage BAY CANADA 49° http://www.charts.noaa.gov/InteractiveCatalog/nrnc.shtml UNITED STATES S T R Blaine 125° A I T O F G E O R V ANCOUVER ISLAND G (CANADA) I A 18431 18432 18424 Bellingham A S S Y P B 18460 A R 18430 E N D L U L O I B N G Orcas Island H A M B A Y H A R O San Juan Island S T 48°30' R A S I Lopez Island Anacortes T 18465 T R A I Victoria T O F 18433 18484 J 18434 U A N D E F U C Neah Bay A 18427 18429 SKAGIT BAY 18471 A D M I R A L DUNGENESS BAY T 18485 18468 Y I N Port Townsend L E T Port Angeles W ASHINGTON 48° 31 MAY 2020 31 MAY 31 MAY 2020 U.S. Coast Pilot 7, Chapter 12 ¢ 489 Strait of Juan De Fuca and Georgia, Washington (1) thick weather, because of strong and irregular currents, ENC - extreme caution and vigilance must be exercised. Chart - 18400 Navigators not familiar with these waters should take a pilot. (2) This chapter includes the Strait of Juan de Fuca, (7) Sequim Bay, Port Discovery, the San Juan Islands and COLREGS Demarcation Lines its various passages and straits, Deception Pass, Fidalgo (8) The International Regulations for Preventing Island, Skagit and Similk Bays, Swinomish Channel, Collisions at Sea, 1972 (72 COLREGS) apply on all the Fidalgo, Padilla, and Bellingham Bays, Lummi Bay, waters of the Strait of Juan de Fuca, Haro Strait, and Strait Semiahmoo Bay and Drayton Harbor and the Strait of of Georgia.