Supporting Information
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

FTSE Japan ESG Low Carbon Select
2 FTSE Russell Publications 19 August 2021 FTSE Japan ESG Low Carbon Select Indicative Index Weight Data as at Closing on 30 June 2021 Constituent Index weight (%) Country Constituent Index weight (%) Country Constituent Index weight (%) Country ABC-Mart 0.01 JAPAN Ebara 0.17 JAPAN JFE Holdings 0.04 JAPAN Acom 0.02 JAPAN Eisai 1.03 JAPAN JGC Corp 0.02 JAPAN Activia Properties 0.01 JAPAN Eneos Holdings 0.05 JAPAN JSR Corp 0.11 JAPAN Advance Residence Investment 0.01 JAPAN Ezaki Glico 0.01 JAPAN JTEKT 0.07 JAPAN Advantest Corp 0.53 JAPAN Fancl Corp 0.03 JAPAN Justsystems 0.01 JAPAN Aeon 0.61 JAPAN Fanuc 0.87 JAPAN Kagome 0.02 JAPAN AEON Financial Service 0.01 JAPAN Fast Retailing 3.13 JAPAN Kajima Corp 0.1 JAPAN Aeon Mall 0.01 JAPAN FP Corporation 0.04 JAPAN Kakaku.com Inc. 0.05 JAPAN AGC 0.06 JAPAN Fuji Electric 0.18 JAPAN Kaken Pharmaceutical 0.01 JAPAN Aica Kogyo 0.07 JAPAN Fuji Oil Holdings 0.01 JAPAN Kamigumi 0.01 JAPAN Ain Pharmaciez <0.005 JAPAN FUJIFILM Holdings 1.05 JAPAN Kaneka Corp 0.01 JAPAN Air Water 0.01 JAPAN Fujitsu 2.04 JAPAN Kansai Paint 0.05 JAPAN Aisin Seiki Co 0.31 JAPAN Fujitsu General 0.01 JAPAN Kao 1.38 JAPAN Ajinomoto Co 0.27 JAPAN Fukuoka Financial Group 0.01 JAPAN KDDI Corp 2.22 JAPAN Alfresa Holdings 0.01 JAPAN Fukuyama Transporting 0.01 JAPAN Keihan Holdings 0.02 JAPAN Alps Alpine 0.04 JAPAN Furukawa Electric 0.03 JAPAN Keikyu Corporation 0.02 JAPAN Amada 0.01 JAPAN Fuyo General Lease 0.08 JAPAN Keio Corp 0.04 JAPAN Amano Corp 0.01 JAPAN GLP J-REIT 0.02 JAPAN Keisei Electric Railway 0.03 JAPAN ANA Holdings 0.02 JAPAN GMO Internet 0.01 JAPAN Kenedix Office Investment Corporation 0.01 JAPAN Anritsu 0.15 JAPAN GMO Payment Gateway 0.01 JAPAN KEWPIE Corporation 0.03 JAPAN Aozora Bank 0.02 JAPAN Goldwin 0.01 JAPAN Keyence Corp 0.42 JAPAN As One 0.01 JAPAN GS Yuasa Corp 0.03 JAPAN Kikkoman 0.25 JAPAN Asahi Group Holdings 0.5 JAPAN GungHo Online Entertainment 0.01 JAPAN Kinden <0.005 JAPAN Asahi Intecc 0.01 JAPAN Gunma Bank 0.01 JAPAN Kintetsu 0.03 JAPAN Asahi Kasei Corporation 0.26 JAPAN H.U. -

Mass Spectrometer Business Presentation Materials
Mass Spectrometer Business Presentation Materials Hiroto Itoi, Corporate Officer Deputy General Manager of the Analytical & Measuring Instruments Division Shimadzu Corporation Jul. 3, 2018 Contents I. Introduction • Expansion of Mass Spectrometry ………………………………………………………………… p.3 • History of Shimadzu's Growth in Mass Spectrometry …………………………………………… p.5 II. Overview of Mass Spectrometers • Operating Principle, Demand Trends, and Vendors ……………………………………………… p.9 • Mass Spectra ………………………………………………………………………………………… p.10 • Configuration of Mass Spectrometers …………………………………………………………… p.11 • Ionization …………………………………………………………………………………………… p.12 • Mass Separation …………………………………………………………………………………… p.14 III. Shimadzu's Mass Spectrometer Business • Product Type ………………………………………………………………………………………… p.17 • Application Software ………………………………………………………………………………… p.18 • Growth Strategy for Mass Spectrometer Business ……………………………………………… p.19 • Expand/Improve Product Lines …………………………………………………………………… p.20 • Measures to Expand Application Fields …………………………………………………………… p.24 • Measures to Automate Data Processing Using AI ……………………………………………… p.25 IV. Summary • Future Direction ……………………………………………………………………………………… p.26 July 2018 Mass Spectrometer Business Presentation Materials 2 I. Introduction Expansion of Mass Spectrometry (1) Why Mass Spectrometry? Mass spectrometry is able to analyze a wide variety of compounds with high accuracy and high efficiency (simultaneous multicomponent analysis). It offers superior characteristics that are especially beneficial in the following fields, -
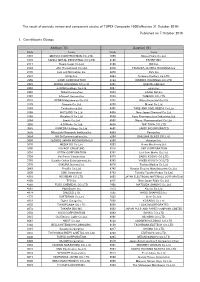
Published on 7 October 2016 1. Constituents Change the Result Of
The result of periodic review and component stocks of TOPIX Composite 1500(effective 31 October 2016) Published on 7 October 2016 1. Constituents Change Addition( 70 ) Deletion( 60 ) Code Issue Code Issue 1810 MATSUI CONSTRUCTION CO.,LTD. 1868 Mitsui Home Co.,Ltd. 1972 SANKO METAL INDUSTRIAL CO.,LTD. 2196 ESCRIT INC. 2117 Nissin Sugar Co.,Ltd. 2198 IKK Inc. 2124 JAC Recruitment Co.,Ltd. 2418 TSUKADA GLOBAL HOLDINGS Inc. 2170 Link and Motivation Inc. 3079 DVx Inc. 2337 Ichigo Inc. 3093 Treasure Factory Co.,LTD. 2359 CORE CORPORATION 3194 KIRINDO HOLDINGS CO.,LTD. 2429 WORLD HOLDINGS CO.,LTD. 3205 DAIDOH LIMITED 2462 J-COM Holdings Co.,Ltd. 3667 enish,inc. 2485 TEAR Corporation 3834 ASAHI Net,Inc. 2492 Infomart Corporation 3946 TOMOKU CO.,LTD. 2915 KENKO Mayonnaise Co.,Ltd. 4221 Okura Industrial Co.,Ltd. 3179 Syuppin Co.,Ltd. 4238 Miraial Co.,Ltd. 3193 Torikizoku co.,ltd. 4331 TAKE AND GIVE. NEEDS Co.,Ltd. 3196 HOTLAND Co.,Ltd. 4406 New Japan Chemical Co.,Ltd. 3199 Watahan & Co.,Ltd. 4538 Fuso Pharmaceutical Industries,Ltd. 3244 Samty Co.,Ltd. 4550 Nissui Pharmaceutical Co.,Ltd. 3250 A.D.Works Co.,Ltd. 4636 T&K TOKA CO.,LTD. 3543 KOMEDA Holdings Co.,Ltd. 4651 SANIX INCORPORATED 3636 Mitsubishi Research Institute,Inc. 4809 Paraca Inc. 3654 HITO-Communications,Inc. 5204 ISHIZUKA GLASS CO.,LTD. 3666 TECNOS JAPAN INCORPORATED 5998 Advanex Inc. 3678 MEDIA DO Co.,Ltd. 6203 Howa Machinery,Ltd. 3688 VOYAGE GROUP,INC. 6319 SNT CORPORATION 3694 OPTiM CORPORATION 6362 Ishii Iron Works Co.,Ltd. 3724 VeriServe Corporation 6373 DAIDO KOGYO CO.,LTD. 3765 GungHo Online Entertainment,Inc. -

C054-E076 up Series
C054-E076 UniBloc Performance Balances UP Series 836HULHV 8QL%ORFū 3HUIRUPDQFH%DODQFHV Superior Response Signicantly Increases Weighing Efciency +LJK6SHHG The display response time for weighing minute quantities (from about ten times the minimum display value) is about one second. That signicantly improves weighing efciency. 7RXJKQHVV Using the highly impact-resistant UniBloc sensor minimizes downtime due to malfunctions. 6WUHVV)UHH The STABLO™-AP ionizer (optional) eliminates static electricity effects to provide highly reliable measurements without any time-consuming steps. Large Pan with 0.01 g Large Pan with 0.1 g Minimum Display Value Minimum Display Value • UP2202X • UP2202Y • UP4201X • UP4201Y • UP4202X • UP4202Y • UP8201X • UP8201Y • UP6202X • UP6202Y Movie that explains UP-series features in easy-to-understand terms. https://www.shimadzu.com/an/balance/analytical/up-6.html Small Pan with 0.001 g Small Pan with 0.01 g Minimum Display Value Minimum Display Value • UP223X • UP223Y • UP423X • UP423Y • UP422X • UP422Y • UP623X • UP623Y • UP823X • UP823Y • UP822X • UP822Y • UP1023X • UP1023Y +LJK6SHHG High-speed weighing signicantly improves efciency of weighing operations. Signicantly Improved Response Provides Fastest Response Performance in Its Class Advanced digital control technology shortens display response times to about one ninth of previous values when weighing minute quantities, which signicantly improves work efciency. Response for Weighing Minute Quantities Large pan model with 0.01 g minimum display value Small pan model with 0.001 g minimum display value Previous 9 model 9× UP 1 Speedy Faster Response (vs. previous Shimadzu model) 0246810 Time (sec.) Measurement Conditions: Large pan model with 0.01 g minimum display value and 0.1 g load Small pan model with 0.001 g minimum display value and 0.01 g load UP-Wind Break (large pan windbreak) (only for large pan models with 0.01 g minimum display value) Newly designed UP-Wind Break included standard. -

Proposal of a Data Processing Guideline for Realizing Automatic Measurement Process with General Geometrical Tolerances and Contactless Laser Scanning
Proposal of a data processing guideline for realizing automatic measurement process with general geometrical tolerances and contactless laser scanning 2018/4/4 Atsuto Soma Hiromasa Suzuki Toshiaki Takahashi Copyright (c)2014, Japan Electronics and Information Technology Industries Association, All rights reserved. 1 Contents • Introduction of the Project • Problem Statements • Proposed Solution – Proposal of New General Geometric Tolerance (GGT) – Data Processing Guidelines for point cloud • Next Steps Copyright (c)2014, Japan Electronics and Information Technology Industries Association, All rights reserved. 2 Contents • Introduction of the Project • Problem Statements • Proposed Solution – Proposal of New General Geometric Tolerance (GGT) – Data Processing Guidelines for Point Cloud • Next Steps Copyright (c)2014, Japan Electronics and Information Technology Industries Association, All rights reserved. 3 Introduction of JEITA What is JEITA? The objective of the Japan Electronics and Information Technology Industries Association (JEITA) is to promote healthy manufacturing, international trade and consumption of electronics products and components in order to contribute to the overall development of the electronics and information technology (IT) industries, and thereby to promote further Japan's economic development and cultural prosperity. JEITA’s Policy and Strategy Board > Number of full members: 279> Number of associate members: 117(as of May 13, 2014) - Director companies and chair/subchair companies - Policy director companies (alphabetical) Fujitsu Limited (chairman Masami Yamamoto) Asahi Glass Co., Ltd. Nichicon Corporation Sharp Corporation Azbil Corporation IBM Japan, Ltd. Hitachi, Ltd. Advantest Corporation Nippon Chemi-Con Corporation Panasonic Corporation Ikegami Tsushinki Co., Ltd. Japan Aviation Electronics Industry, Ltd. SMK Corporation Mitsubishi Electric Corporation Nihon Kohden Corporation Omron Corporation NEC Corporation JRC Nihon Musen Kyocera Corporation Sony Corporation Hitachi Metals, Ltd KOA Corporation Fuji Xerox Co., Ltd. -

Facilitation of Information Transfer on Chemicals in Products
Facilitation of Information Transfer on Chemicals in Products The Ministry of Economy, Trade and Industry (METI) developed ‘chemSHERPA’ [kémʃéərpə] as a new information transfer scheme for chemicals in products throughout their supply chains. METI hopes that the dissemination of chemSHERPA may contribute to reduce the workload of both providers and recipients of the information. From the beginning of the development of chemSHERPA, METI has been in communication with international bodies such as the IEC and the IPC, etc., with the aim of developing chemSHERPA into not only a Japanese standard but also an International standard. To make it a de-facto standard, METI has introduced this scheme to international organizations and governments of other countries for their active use. The Joint Article Management Promotion Consortium (JAMP) is a governing body for chemSHERPA from April 2016 and see a shift to chemSHERPA. We believe many companies are preparing towards implementing chemSHERPA. Based on the efforts mentioned above, the following companies and company groups have agreed with the dissemination of chemSHERPA, and METI will continue to work with JAMP and companies to spread the use of chemSHERPA to internal as well as external supply chains as needed.(Please contact us if any company or company group has interest in putting its name below.) It should be noted, the use of the provision of data entry support tools is free of charge in principle with the aim of promoting wider use of chemSHERPA. [Contact information] Chemical Management Policy Division Manufacturing Industries Bureau Ministry of Economy, Trade and Industry [email protected] 03-3501-0080 (direct) 03-3501-1511 (ex. -

Facilitation of Information Transfer on Chemicals in Products
Facilitation of Information Transfer on Chemicals in Products The Ministry of Economy, Trade and Industry (METI) has developed ‘chemSHERPA’ [kémʃéərpə] as a new information transfer scheme for chemicals in products throughout their supply chains. METI hopes that the dissemination of chemSHERPA may contribute to reduce the workload of both providers and recipients of the information. From the beginning of the development of chemSHERPA, METI has been in communication with international bodies such as the IEC and the IPC, etc., with the aim of developing chemSHERPA into not only a Japanese standard but also an International standard. To make it a de-facto standard, METI has introduced this scheme to international organizations and governments of other countries for their active use. The Joint Article Management Promotion Consortium (JAMP) will be a governing body for chemSHERPA from April 2016 and METI expects to see an orderly, step-by-step shift to chemSHERPA over the two years transition period. Accordingly JAMP has a plan to finish renewing the substances list in the existing JAMP scheme by the end of March of 2018. We believe many companies will begin advance preparations towards implementing chemSHERPA. Based on the efforts mentioned above, the following companies and company groups have agreed with the dissemination of chemSHERPA, and METI will continue to work with companies to spread the use of chemSHERPA to internal as well as external supply chains.(Please contact us if any company or company group has interest in putting its name below.) It should be noted, the use of the provision of data entry support tools is free of charge in principle with the aim of promoting wider use of chemSHERPA. -

Visible-Light-Induced Photocatalytic Benzene/Cyclohexane Cross
Electronic Supplementary Material (ESI) for Catalysis Science & Technology. This journal is © The Royal Society of Chemistry 2018 Supporting Information Visible-light-induced Photocatalytic Benzene/Cyclohexane Cross-coupling Utilizing a Ligand-to-metal Charge Transfer Benzene Complex Adsorbed on Titanium Oxides A. Yamamoto,*ab T. Ohara,a and H. Yoshida*ab a Graduate School of Human and Environmental Studies, Kyoto University, Kyoto, 606-8501, Japan. b Elements Strategy Initiative for Catalysts and Batteries (ESICB), Kyoto University, Kyoto 615-8520, Japan. E-mail: [email protected] [email protected] S1 2 –1 Material and Synthesis. An anatase TiO2 powder (JRC-TIO-8, 338 m g ) was donated from the Catalysis Society of Japan. PdCl2 (Kishida, 99%), H2PtCl6·6H2O (Wako, 99.9%), RhCl3·3H2O (Kishida, 99%), HAuCl4.4H2O (Kishida, 99.9%), Ag(NO3) (Kishida, 99%), Ni(NO3)2·6H2O (Wako, 98%) or Co(NO3)2·6H2O (Nacalai tesque, 98%) were used as a metal precursor of co-catalysts, and co-catalyst was loaded on the TiO2 powder by a photodeposition method. The TiO2 powder was dispersed in a methanol/water solution (25% v/v, 400 mL) containing the metal precursor (0.1 wt%), and irradiated using a xenon lamp (Perkin Elmer PE300BUV) with stirring for 30 min. The light intensity was 50 mW cm–2 when measured at 365 ± 20 nm wavelength using a UV radiometer (Topcon, UVR-2, UD-36). After the filtration, the sample was washed with ion-exchanged water, and then was dried at 323 K. The catalyst is referred to as M/TiO2 (M = Rh, Pt, Au, Pd, Ag, Ni, and Co). -
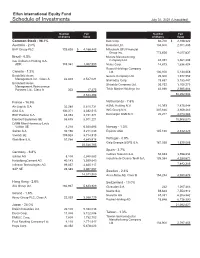
Schedule of Investments July 31, 2021 (Unaudited)
Elfun International Equity Fund Schedule of Investments July 31, 2021 (Unaudited) Number Fair Number Fair of Shares Value of Shares Value Common Stock - 96.1% Kao Corp. 36,700 $ 2,198,322 Australia - 2.0% Komatsu Ltd. 104,800 2,701,405 BHP Group PLC 129,626 $ 4,188,443 Mitsubishi UFJ Financial Group Inc. 773,626 4,077,837 Brazil - 0.5% Murata Manufacturing Itau Unibanco Holding S.A. Company Ltd. 48,091 3,961,208 ADR 183,361 1,057,993 Nidec Corp. 14,673 1,636,424 Recruit Holdings Company Canada - 1.2% Ltd. 100,100 5,134,058 Brookfield Asset Secom Company Ltd. 26,300 1,977,952 Management Inc., Class A 46,839 2,527,021 Shimadzu Corp. 78,657 3,153,447 Brookfield Asset Shiseido Company Ltd. 56,762 3,768,273 Management Reinsurance Partners Ltd., Class A 323 17,475 Tokio Marine Holdings Inc. 62,898 2,985,864 2,544,496 50,292,594 France - 16.9% Netherlands - 7.6% Air Liquide S.A. 32,280 5,610,731 ASML Holding N.V. 10,393 7,875,044 AXA S.A. 130,371 3,382,513 ING Groep N.V. 307,846 3,959,263 BNP Paribas S.A. 68,053 4,151,871 Koninklijke DSM N.V. 20,217 4,074,263 Dassault Systemes SE 55,675 3,071,221 15,908,570 LVMH Moet Hennessy Louis Vuitton SE 8,210 6,560,695 Norway - 1.3% Safran S.A. 32,196 4,211,033 Equinor ASA 145,138 2,832,826 Vivendi SE 109,924 3,714,913 Worldline S.A. -

ERADICATING FORCED LABOR in ELECTRONICS: What Do Company Statements Under the UK Modern Slavery Act Tell Us?
ERADICATING FORCED LABOR IN ELECTRONICS: What do company statements under the UK Modern Slavery Act tell us? March 2018 TABLE OF CONTENTS Executive Summary 3 Introduction: Forced Labor Risks in Electronics Supply Chains 5 Company Selection 7 Compliance with the UK Modern Slavery Act 8 What Actions Have Companies Taken? 11 Findings by Theme: Gaps and Good Practice Examples 13 1. Commitment and Governance, including Training 13 2. Traceability and Risk Assessment 14 3. Purchasing Practices 15 4. Recruitment 16 5. Worker Voice 17 6. Monitoring 18 7. Remedy 19 Recommendations for Electronic Companies 20 Appendix 1: ICT Companies Required to Report under the UK Modern Slavery Act 21 Appendix 2: Benchmark Methodology - Information and Communications Technology 25 2 KnowTheChain TABLE OF CONTENTS EXECUTIVE SUMMARY The information and communications technology sector medium-size global ICT companies and identified 102 (ICT) is at high risk of forced labor. A significant number of companies from Asia, Europe, and the United States required workers in electronics supply chains are migrant workers to report under the Modern Slavery Act. We reached out to 23 who are particularly vulnerable to exploitation. The US of those companies that had not published a statement. We Department of Labor lists China and Malaysia as countries also assessed compliance among published statements with where electronics may be produced using forced labor. In the minimum requirements of the Modern Slavery Act: the fact, a 2014 Verité study found that nearly a third of migrant statement must be linked on the homepage of the company’s workers in Malaysia’s electronics sector are in situations of website, signed by a director or equivalent, and approved by forced labor.1 the board. -

Weston Was the Icon of Meters in Japan
2007 IEEE Conference on the History of Electric Power Weston was the Icon of Meters in Japan Eiju MATSUMOTO* Society of Historical Metrology, Japan Abstract Weston Electrical Instrument Corporation was founded in 1888 and began to manufacture portable direct-current meters. At that time, the electricity power industry emerged in Japan, and in 1888, the Institute of Electrical Engineers of Japan (IEEJ) was also established. Many Weston meters were imported to Japan and used by government institutions and universities. Japanese meter manufacturers were established around 1900 and began to manufacture meters similar to Weston's meters. It took a while for them to become able to make meters that were comparable to Weston's. On the other hand, it took a long time for Japanese users to acknowledge that the performance of domestic meters achieved the level of Weston's. In other words, Weston's meters were the icon of meters for Japanese users and maintained their superiority status for a long time in Japan. The following meters were Weston's specialties: 1) Laboratory Standard Instrument (accuracy of 0.1%) 2) Portable Precision Instrument (accuracy of 0.25%) 3) Photographic Exposure Meter Weston's superiority status in Japan continued until around 1950, after World War II. The arrival of digital instruments, however, changed the situation radically. Although the company had excellent patents, such as one for the dual-slope A/D converter, Weston Corporation passed on the opportunity to survive the competition in the meter industry. 1. Weston Electrical Instrument and voltage [2]. Corporation and Japan Many Japanese meter manufacturers were Edward Weston (1850-1936) completed a founded before and after World War I (1914 - portable precision electrical instrument in 1918), because the war made it difficult for 1888 and founded Weston Electrical Japan to export and import products. -

Formation and Suppression of Hydrogen Blisters in Tunnelling
www.nature.com/scientificreports OPEN Formation and suppression of hydrogen blisters in tunnelling oxide passivating contact for crystalline silicon solar cells Sungjin Choi1, Ohmin Kwon2, Kwan Hong Min1,3, Myeong Sang Jeong1,3, Kyung Taek Jeong1, Min Gu Kang1, Sungeun Park1, Kuen Kee Hong4, Hee-eun Song1 ✉ & Ka-Hyun Kim2,5 ✉ The formation of hydrogen blisters in the fabrication of tunnelling oxide passivating contact (TOPCon) solar cells critically degrades passivation. In this study, we investigated the formation mechanism of blisters during the fabrication of TOPCons for crystalline silicon solar cells and the suppression of such blisters. We tested the efects of annealing temperature and duration, surface roughness, and deposition temperature on the blister formation, which was suppressed in two ways. First, TOPCon fabrication on a rough surface enhanced adhesion force, resulting in reduced blister formation after thermal annealing. Second, deposition or annealing at higher temperatures resulted in the reduction of hydrogen in the flm. A sample fabricated through low-pressure chemical vapor deposition at 580 °C was free from silicon–hydrogen bonds and blisters after the TOPCon structure was annealed. Remarkably, samples after plasma-enhanced chemical vapor deposition at 300, 370, and 450 °C were already blistered in the as-deposited state, despite low hydrogen contents. Analysis of the hydrogen incorporation, microstructure, and deposition mechanism indicate that in plasma-enhanced chemical vapor deposition (PECVD) deposition, although the increase of substrate temperature reduces the hydrogen content, it risks the increase of porosity and molecular-hydrogen trapping, resulting in even more severe blistering. Te conversion efciency of industrial crystalline silicon (c-Si) solar cells has continuously improved and is about to reach 22%1.