Monkeypox Nov 2017 2017
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Uveal Involvement in Marburg Virus Disease B
Br J Ophthalmol: first published as 10.1136/bjo.61.4.265 on 1 April 1977. Downloaded from British Journal of Ophthalmology, 1977, 61, 265-266 Uveal involvement in Marburg virus disease B. S. KUMING AND N. KOKORIS From the Department of Ophthalmology, Johannesburg General Hospital and University of the Witwatersrand SUMMARY The first reported case of uveal involvement in Marburg virus disease is described. 'Ex Africa semper aliquid novi'. Two outbreaks of Marburg virus disease have been Rhodesia and had also been constantly at his documented. The first occurred in Marburg and bedside till his death. Lassa fever was suspected and Frankfurt, West Germany, in 1967 (Martini, 1969) she was given a unit of Lassa fever convalescent and the second in Johannesburg in 1975 (Gear, serum when she became desperately ill on the fifth 1975). This case report describes the third patient day. She also developed acute pancreatitis. Within in the Johannesburg outbreak, who developed an 52 hours she made a dramatic and uneventful anterior uveitis. The cause of the uveitis was proved recovery. Her illness mainly affected the haema- to be the Marburg virus by identiying it in a tissue topoietic, hepatic, and pancreatic systems. culture of her aqueous fluid. The subject of this report was a nurse who had helped to nurse patients 1 and 2. Nine days after the Case report death ofthe first patient she presented with lower back pain and high fever. She developed hepatitis, a mild Before describing the case history of the patient the disseminated intravascular coagulation syndrome, events leading to her contracting the disease must successfully treated with heparin, and the classical be briefly described. -

Poxvirus and Rabies Branch (PRB) Fact Sheet
Poxvirus & Rabies Branch (PRB) The epidemiologists, laboratory scientists, and public health professionals in PRB are responsible for surveillance, control, and prevention of illness and death due to poxviruses and rabies. Our Work Laboratory Reference, Diagnostics, & Training PRB’s provides laboratory reference, diagnostic services, and trainings to state and local health departments, the Department of Defense (DOD), international organizations, and the Laboratory Response Network (over 150 labs across the U.S. prepared to respond to bioterrorism). PRB collaborates with the private sector to develop and evaluate novel diagnostic assays, therapeutics, and vaccines for rabies and pox-related viruses. The branch maintains high-containment laboratories (BSL3 &4) to safely conduct public health research. Research & Surveillance PRB conducts research studies on the microbiology, molecular biology, pathogenesis (disease process), ecology, and evolution of Our Pathogens poxviruses and rabies. The branch monitors the incidence rates of PRB’s Poxvirus Team provides subject matter these diseases in the U.S. and internationally. expertise on: Expert Consultation Orthopoxviruses, including PRB provides consultation regarding poxvirus and rabies- Smallpox associated diseases and their diagnoses, prevention, control, and Monkeypox treatments. Assistance is offered to healthcare providers, academic Cowpox institutions, state and local health departments, other government Vaccinia Virus agencies, and the general public. PRB collaborates with non- governmental organizations to develop risk communication Parapoxviruses, including tools for disease prevention. The branch provides training on Orf Virus simple surveillance techniques and trains healthcare workers on monkeypox in the Democratic Republic of the Congo (DRC). PRB Pseudocowpox collaborates with the national government of Haiti on a rabies surveillance and control program which includes health education, enhanced diagnostics, surveillance, and an extensive dog vaccination campaign. -

Chikungunya Fever: Epidemiology, Clinical Syndrome, Pathogenesis
Antiviral Research 99 (2013) 345–370 Contents lists available at SciVerse ScienceDirect Antiviral Research journal homepage: www.elsevier.com/locate/antiviral Review Chikungunya fever: Epidemiology, clinical syndrome, pathogenesis and therapy ⇑ Simon-Djamel Thiberville a,b, , Nanikaly Moyen a,b, Laurence Dupuis-Maguiraga c,d, Antoine Nougairede a,b, Ernest A. Gould a,b, Pierre Roques c,d, Xavier de Lamballerie a,b a UMR_D 190 ‘‘Emergence des Pathologies Virales’’ (Aix-Marseille Univ. IRD French Institute of Research for Development EHESP French School of Public Health), Marseille, France b University Hospital Institute for Infectious Disease and Tropical Medicine, Marseille, France c CEA, Division of Immuno-Virologie, Institute of Emerging Diseases and Innovative Therapies, Fontenay-aux-Roses, France d UMR E1, University Paris Sud 11, Orsay, France article info abstract Article history: Chikungunya virus (CHIKV) is the aetiological agent of the mosquito-borne disease chikungunya fever, a Received 7 April 2013 debilitating arthritic disease that, during the past 7 years, has caused immeasurable morbidity and some Revised 21 May 2013 mortality in humans, including newborn babies, following its emergence and dispersal out of Africa to the Accepted 18 June 2013 Indian Ocean islands and Asia. Since the first reports of its existence in Africa in the 1950s, more than Available online 28 June 2013 1500 scientific publications on the different aspects of the disease and its causative agent have been pro- duced. Analysis of these publications shows that, following a number of studies in the 1960s and 1970s, Keywords: and in the absence of autochthonous cases in developed countries, the interest of the scientific commu- Chikungunya virus nity remained low. -

Viral Hemorrhagic Fevers and Bioterrorism
What you need to know about . Viral Hemorrhagic Fevers and Bioterrorism What are viral hemorrhagic fevers? How are viral hemorrhagic fevers Viral hemorrhagic fevers (VHFs) are a spread? group of illnesses caused by several distinct In nature, viruses causing hemorrhagic fever families of viruses. In general the term typically are passed from mice, rats, fleas “viral hemorrhagic fever” describes severe and ticks to humans. People can be infected problems affecting several organ systems when they come in contact with urine, fecal in the body. Typically, the entire system of ma�er, saliva or other body fluids from blood vessels is damaged, and the body has infected rodents. Fleas and ticks transmit the problems regulating itself. Symptoms o�en viruses when they bite a person or when a include bleeding, but the bleeding itself is person crushes a tick. Hosts for some viruses rarely life-threatening. VHFs are caused by such as Ebola and Marburg are not known. viruses of four families: Some viruses such as Ebola, Marburg and Lassa can be spread from person to person • Arenavirus including Lassa fever and by direct contact with infected blood or Argentine, Bolivian, Brazilian and organs or indirectly through contact with Venezuelan hemorrhagic fevers; objects such as syringes or needles that are • Filovirus including Ebola and Marburg; contaminated with infected body fluids. • Bunyavirus including Hantavirus and Ri� Valley Fever; What are the symptoms? • Flavivirus including yellow fever and Symptoms vary with the different virus dengue fever. families, but first signs o�en include sudden fever, weakness, muscle pain, tiredness, Can viral hemorrhagic fevers be used headache and sore throat. -

Dengue Fever in Senegal 6 - 7 Ongoing Events Ebola Virus Disease in the Democratic Republic of the Congo Humanitarian Crisis in Cameroon
Overview Contents This Weekly Bulletin focuses on selected acute public health emergencies occurring in the WHO African Region. The WHO Health Emergencies Programme is currently monitoring 58 events in the region. This week’s edition covers key new and ongoing events, including: 2 Overview Hepatitis E in Central African Republic 3 - 5 New events Monkeypox in Central African Republic Dengue fever in Senegal 6 - 7 Ongoing events Ebola virus disease in the Democratic Republic of the Congo Humanitarian crisis in Cameroon. 8 Summary of major issues challenges and For each of these events, a brief description, followed by public health proposed actions measures implemented and an interpretation of the situation is provided. 9 All events currently A table is provided at the end of the bulletin with information on all new and being monitored ongoing public health events currently being monitored in the region, as well as events that have recently been closed. Major issues and challenges include: The Ebola virus disease (EVD) outbreak in the Democratic Republic of the Congo has reached a critical juncture, marked by a precarious security situation, persistence of pockets of community resistance/ mistrust and expanding geographical spread of the disease. During the reporting week, there was an incident involving a response team performing burial activity in Butembo. This came barely days following a widespread community strike (“ville morte”) in Beni and several towns, and an earlier armed attack in Beni. These incidents severely disrupted most outbreak control interventions. Meanwhile, EVD cases have been confirmed in new areas with worse insecurity and in close proximity to the border with Uganda. -

Imported Monkeypox, Singapore
DISPATCHES Imported Monkeypox, Singapore Sarah Ee Fang Yong, Oon Tek Ng, Zheng Jie Marc Ho, Tze Minn Mak, Kalisvar Marimuthu, Shawn Vasoo, Tsin Wen Yeo, Yi Kai Ng, Lin Cui, Zannatul Ferdous, Po Ying Chia, Bryan Jun Wei Aw, Charmaine Malenab Manauis, Constance Khia Ki Low, Guanhao Chan, Xinyi Peh, Poh Lian Lim, Li Ping Angela Chow, Monica Chan, Vernon Jian Ming Lee, Raymond Tzer Pin Lin, Mok Kwee Derrick Heng, Yee Sin Leo In May 2019, we investigated monkeypox in a traveler and public health management for this case, together from Nigeria to Singapore. The public health response with lessons learned and implications for control. included rapid identification of contacts, use of quaran- tine, and postexposure smallpox vaccination. No sec- The Case ondary cases were identified. Countries should develop On May 8, 2019, monkeypox was laboratory-con- surveillance systems to detect emerging infectious dis- firmed in a 38-year-old man from Nigeria who had eases globally. traveled to Singapore. The man resided in Delta State, Nigeria, but had attended a wedding in Ebonyi State onkeypox is a zoonosis endemic to West and during April 21–23, where he reported ingestion of MCentral Africa; human cases were first report- barbecued bushmeat that might have been contami- ed in 1970 (1). An outbreak ongoing in Nigeria since nated. He did not handle raw meat and had no expo- 2017 is the largest documented (2). Exported cases in sure to wild animals or their products. He held an ad- the United Kingdom and Israel were reported from ministrative job and reported no contact with rodents travelers infected in Nigeria in 2018 (3,4). -

Viral Hemorrhagic Fevers (Lassa, Marburg, Ebola, Crimean-Congo, and Other Emerging Viruses)
Viral Hemorrhagic Fevers (Lassa, Marburg, Ebola, Crimean-Congo, and other emerging viruses) What Are They? Viral hemorrhagic fevers are a group of illnesses causes by several viruses. These viruses affect multiple organs in the body by damaging the vascular (blood vessel) system. The bleeding or hemorrhaging caused by the virus is not usually life threatening but damage to organ systems in the body can range from mild to deadly. The viruses responsible for this type of illness include Lassa, Marburg, Ebola, and Crimean-Congo hemorrhagic fever. How can you get it? These emerging viral hemorrhagic fevers are presumed to be animal borne (zoonotic) and can be transmitted to humans through contact. Infected humans can spread the virus to each other through contact with contaminated objects or blood. The risk of acquiring these diseases is typically restricted to the geographic regions where the virus is found. Given global travel, rare cases have been reported outside of the host region. These rare cases are probably the greatest form of the occupational threat to fire fighters. Lassa Associated with specific rodents Found in West Africa Marburg Transmitted by African fruit bat Found in Africa Ebola Transmitted by unknown animal Found in Africa Crimean-Congo Tick-borne virus Found in Africa, Asia, Europe What are the symptoms? The time to develop symptoms varies by virus but is between 2 to 21 days after exposure to the Ebola virus. The signs and symptoms of viral hemorrhagic fever vary depending on the virus but include: Flu-like symptoms o Fever o Fatigue o Muscle aches Exhaustion Nausea and/or vomiting Abdominal pain Shock Seizures Delirium Bleeding Organ failure The most common complication of Lassa fever is deafness. -

1 Lujo Viral Hemorrhagic Fever: Considering Diagnostic Capacity And
1 Lujo Viral Hemorrhagic Fever: Considering Diagnostic Capacity and 2 Preparedness in the Wake of Recent Ebola and Zika Virus Outbreaks 3 4 Dr Edgar Simulundu1,, Prof Aaron S Mweene1, Dr Katendi Changula1, Dr Mwaka 5 Monze2, Dr Elizabeth Chizema3, Dr Peter Mwaba3, Prof Ayato Takada1,4,5, Prof 6 Guiseppe Ippolito6, Dr Francis Kasolo7, Prof Alimuddin Zumla8,9, Dr Matthew Bates 7 8,9,10* 8 9 1 Department of Disease Control, School of Veterinary Medicine, University of Zambia, 10 Lusaka, Zambia 11 2 University Teaching Hospital & National Virology Reference Laboratory, Lusaka, Zambia 12 3 Ministry of Health, Republic of Zambia 13 4 Division of Global Epidemiology, Hokkaido University Research Center for Zoonosis 14 Control, Sapporo, Japan 15 5 Global Institution for Collaborative Research and Education, Hokkaido University, Sapporo, 16 Japan 17 6 Lazzaro Spallanzani National Institute for Infectious Diseases, IRCCS, Rome, Italy 18 7 World Health Organization, WHO Africa, Brazzaville, Republic of Congo 19 8 Department of Infection, Division of Infection and Immunity, University College London, 20 U.K 21 9 University of Zambia – University College London Research & Training Programme 22 (www.unza-uclms.org), University Teaching Hospital, Lusaka, Zambia 23 10 HerpeZ (www.herpez.org), University Teaching Hospital, Lusaka, Zambia 24 25 *Corresponding author: Dr. Matthew Bates 26 Address: UNZA-UCLMS Research & Training Programme, University Teaching Hospital, 27 Lusaka, Zambia, RW1X 1 28 Email: [email protected]; Phone: +260974044708 29 30 2 31 Abstract 32 Lujo virus is a novel old world arenavirus identified in Southern Africa in 2008 as the 33 cause of a viral hemorrhagic fever (VHF) characterized by nosocomial transmission 34 with a high case fatality rate of 80% (4/5 cases). -

To Ebola Reston
WHO/HSE/EPR/2009.2 WHO experts consultation on Ebola Reston pathogenicity in humans Geneva, Switzerland 1 April 2009 EPIDEMIC AND PANDEMIC ALERT AND RESPONSE WHO experts consultation on Ebola Reston pathogenicity in humans Geneva, Switzerland 1 April 2009 © World Health Organization 2009 All rights reserved. The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distin- guished by initial capital letters. All reasonable precautions have been taken by the World Health Organization to verify the information contained in this publication. However, the published material is being distributed without warranty of any kind, either express or implied. The responsibility for the interpretation and use of the material lies with the reader. In no event shall the World Health Organization be liable for damages arising from its use. This publication contains the collective views of an international group of experts and does not necessarily represent the decisions or the policies of the World Health Organization. -

Orthohantaviruses Belonging to Three Phylogroups All Inhibit Apoptosis in Infected Target Cells
www.nature.com/scientificreports OPEN Orthohantaviruses belonging to three phylogroups all inhibit apoptosis in infected target cells Received: 13 July 2018 Carles Solà-Riera1, Shawon Gupta1,2, Hans-Gustaf Ljunggren1 & Jonas Klingström 1 Accepted: 3 December 2018 Orthohantaviruses, previously known as hantaviruses, are zoonotic viruses that can cause hantavirus Published: xx xx xxxx pulmonary syndrome (HPS) and hemorrhagic fever with renal syndrome (HFRS) in humans. The HPS-causing Andes virus (ANDV) and the HFRS-causing Hantaan virus (HTNV) have anti-apoptotic efects. To investigate if this represents a general feature of orthohantaviruses, we analysed the capacity of six diferent orthohantaviruses – belonging to three distinct phylogroups and representing both pathogenic and non-pathogenic viruses – to inhibit apoptosis in infected cells. Primary human endothelial cells were infected with ANDV, HTNV, the HFRS-causing Puumala virus (PUUV) and Seoul virus, as well as the putative non-pathogenic Prospect Hill virus and Tula virus. Infected cells were then exposed to the apoptosis-inducing chemical staurosporine or to activated human NK cells exhibiting a high cytotoxic potential. Strikingly, all orthohantaviruses inhibited apoptosis in both settings. Moreover, we show that the nucleocapsid (N) protein from all examined orthohantaviruses are potential targets for caspase-3 and granzyme B. Recombinant N protein from ANDV, PUUV and the HFRS-causing Dobrava virus strongly inhibited granzyme B activity and also, to certain extent, caspase-3 activity. Taken together, this study demonstrates that six diferent orthohantaviruses inhibit apoptosis, suggesting this to be a general feature of orthohantaviruses likely serving as a mechanism of viral immune evasion. Orthohantaviruses, of the order Bunyavirales and previously known as hantaviruses, are small single-stranded negative-sense RNA viruses with a tri-segmented genome (S, M and L segments) encoding four to fve proteins. -
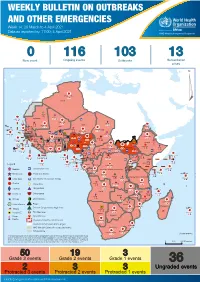
Weekly Bulletin on Outbreaks
WEEKLY BULLETIN ON OUTBREAKS AND OTHER EMERGENCIES Week 14: 29 March to 4 April 2021 Data as reported by: 17:00; 4 April 2021 REGIONAL OFFICE FOR Africa WHO Health Emergencies Programme 0 116 103 13 New event Ongoing events Outbreaks Humanitarian crises 117 622 3 105 Algeria ¤ 36 13 110 0 5 420 164 Mauritania 7 2 10 501 392 110 0 7 0 Niger 17 927 449 Mali 3 334 10 567 0 6 0 2 079 4 4 595 165 Eritrea Cape Verde 38 520 1 037 Chad Senegal 4 918 185 59 0 Gambia 27 0 3 0 17 125 159 9 761 45 Guinea-Bissau 796 17 7 0 Burkina Faso 225 46 215 189 2 963 0 162 593 2 048 Guinea 12 817 150 12 38 397 1 3 662 66 1 1 23 12 Benin 30 0 Nigeria 1 873 71 0 Ethiopia 420 14 481 5 6 188 15 Sierra Leone Togo 3 473 296 53 920 779 52 14 Ghana 5 245 72 Côte d'Ivoire 10 098 108 14 484 479 63 0 40 0 Liberia 17 0 South Sudan Central African Republic 916 2 45 0 25 0 19 670 120 43 180 237 90 287 740 Cameroon 7 0 28 676 137 5 330 13 138 988 2 224 1 952 87 655 2 51 22 43 0 112 12 6 1 488 6 3 988 79 11 187 6 902 102 Equatorial Guinea Uganda 542 8 Sao Tome and Principe 32 11 2 042 85 41 016 335 Kenya Legend 7 100 90 Gabon Congo 18 504 301 Rwanda Humanitarian crisis 2 212 34 22 482 311 Measles 18 777 111 Democratic Republic of the Congo 9 681 135 Burundi 2 964 6 Monkeypox Ebola virus disease Seychelles 27 930 739 1 525 0 420 29 United Republic of Tanzania Lassa fever Skin disease of unknown etiology 189 0 4 084 20 509 21 Cholera Yellow fever 1 349 5 6 257 229 22 631 542 cVDPV2 Dengue fever 88 930 1 220 Comoros Angola Malawi COVID-19 Chikungunya 33 661 1 123 862 0 3 719 146 -

Ebola Virus Disease and Clinical Care Part I: History, Transmission, and Clinical Presentation
Ebola Virus Disease and Clinical Care Part I: History, Transmission, and Clinical Presentation This lecture is on Ebola virus disease (EVD) and clinical care. This is part one of a three-part lecture on this topic. Preparing Healthcare Workers to Work in Ebola Treatment Units (ETUs) in Africa This lecture will focus on EVD in the West African setting. Ebola Virus Disease and Clinical Care: The training and information you receive in this course will Part I: History, Transmission, and Clinical not cover the use of certain interventions such as intubation Presentation or dialysis which are not available in West African Ebola Treatment Units (ETUs). You will need supplemental training This presentation is current as of December 2014. This presentation contains materials from Centers for Disease Control and to care for patients appropriately in countries where advanced Prevention (CDC), Médecins Sans Frontières (MSF), and World Health Organization (WHO). care is available. U.S. Department of Health and Human Services U.S. Department of Health and Human Services Centers for Disease Control and Prevention Centers for Disease Control and Prevention version 12.03.2014 The learning objectives for this lecture are to: Learning Objectives ▶ Describe the routes of Ebola virus transmission Describe the routes of Ebola virus transmission Explain when and how patients are infectious ▶ Explain when and how patients are infectious Describe the clinical features of patients with Ebola ▶ Describe screening criteria for Ebola virus disease Describe the clinical features of patients with Ebola (EVD) used in West Africa Explain how to identify patients with suspected ▶ Describe screening criteria for EVD used in West Africa EVD who present to the ETU ▶ Explain how to identify patients with suspected EVD who present to the ETU This presentation contains materials from CDC, MSF, and WHO 2 A number of different viruses cause viral hemorrhagic fever.