Are We Eating the World's Megafauna to Extinction?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Critically Endangered - Wikipedia
Critically endangered - Wikipedia Not logged in Talk Contributions Create account Log in Article Talk Read Edit View history Critically endangered From Wikipedia, the free encyclopedia Main page Contents This article is about the conservation designation itself. For lists of critically endangered species, see Lists of IUCN Red List Critically Endangered Featured content species. Current events A critically endangered (CR) species is one which has been categorized by the International Union for Random article Conservation status Conservation of Nature (IUCN) as facing an extremely high risk of extinction in the wild.[1] Donate to Wikipedia by IUCN Red List category Wikipedia store As of 2014, there are 2464 animal and 2104 plant species with this assessment, compared with 1998 levels of 854 and 909, respectively.[2] Interaction Help As the IUCN Red List does not consider a species extinct until extensive, targeted surveys have been About Wikipedia conducted, species which are possibly extinct are still listed as critically endangered. IUCN maintains a list[3] Community portal of "possibly extinct" CR(PE) and "possibly extinct in the wild" CR(PEW) species, modelled on categories used Recent changes by BirdLife International to categorize these taxa. Contact page Contents Tools Extinct 1 International Union for Conservation of Nature definition What links here Extinct (EX) (list) 2 See also Related changes Extinct in the Wild (EW) (list) 3 Notes Upload file Threatened Special pages 4 References Critically Endangered (CR) (list) Permanent -
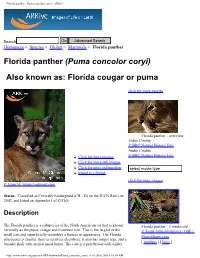
Florida Panther - Puma Concolor Coryi - Arkive
Florida panther - Puma concolor coryi - ARKive Search Homepage > Species > Global > Mammals > Florida panther Florida panther (Puma concolor coryi) Also known as: Florida cougar or puma click for more movies Florida panther - overview Video Credits: © BBC Natural History Unit Audio Credits: © BBC Natural History Unit ● Click for more movies ● Click for more still images ● Click for more information ● Email to a friend click for more images © Lynn M. Stone / naturepl.com Status: Classified as Critically Endangered (CR - D) on the IUCN Red List 2002, and listed on Appendix I of CITES. Description The Florida panther is a subspecies of the North American cat that is known Florida panther - 3 weeks old variously as the puma, cougar and mountain lion. This is the largest of the © Frank Schneidermeyer / OSF / small cats and superficially resembles a lioness in appearance. The Florida Photolibrary.com subspecies is smaller than its relatives elsewhere; it also has longer legs, and a [ medium ] [ large ] broader skull with arched nasal bones. The coat is a pale brown with whiter http://www.arkive.org/species/GES/mammals/Puma_concolor_coryi/ (1 of 2)4/6/2005 8:16:04 AM Florida panther - Puma concolor coryi - ARKive underparts and a black tip at the end of the long tail. Infants have a spotted coat and blue eyes. Florida panthers often have crooked ends to their tails, and whorls of hair on their backs; these are thought not to be characteristic of the subspecies however, and may be signs of inbreeding. Click for more information Florida panther - 5 months old © Bob Bennett / OSF / Photolibrary.com [ medium ] [ large ] © Wildscreen 2004 By using this website you agree to the Terms of Use About ARKive | Competition | Contact | Newsletter | FAQ | Links http://www.arkive.org/species/GES/mammals/Puma_concolor_coryi/ (2 of 2)4/6/2005 8:16:04 AM. -

Megafauna Extinction, Tree Species Range Reduction, and Carbon Storage in Amazonian Forests
Ecography 39: 194–203, 2016 doi: 10.1111/ecog.01587 © 2015 The Authors. Ecography © 2015 Nordic Society Oikos Subject Editor: Yadvinder Mahli. Editor-in-Chief: Nathan J. Sanders. Accepted 27 September 2015 Megafauna extinction, tree species range reduction, and carbon storage in Amazonian forests Christopher E. Doughty, Adam Wolf, Naia Morueta-Holme, Peter M. Jørgensen, Brody Sandel, Cyrille Violle, Brad Boyle, Nathan J. B. Kraft, Robert K. Peet, Brian J. Enquist, Jens-Christian Svenning, Stephen Blake and Mauro Galetti C. E. Doughty ([email protected]), Environmental Change Inst., School of Geography and the Environment, Univ. of Oxford, South Parks Road, Oxford, OX1 3QY, UK. – A. Wolf, Dept of Ecology and Evolutionary Biology, Princeton Univ., Princeton, NJ 08544, USA. – N. Morueta-Holme, Dept of Integrative Biology, Univ. of California – Berkeley, CA 94720, USA. – B. Sandel, J.-C. Svenning and NM-H, Section for Ecoinformatics and Biodiversity, Dept of Bioscience, Aarhus Univ., Ny Munkegade 114, DK-8000 Aarhus C, Denmark. – P. M. Jørgensen, Missouri Botanical Garden, PO Box 299, St Louis, MO 63166-0299, USA. – C. Violle, CEFE UMR 5175, CNRS – Univ. de Montpellier – Univ. Paul-Valéry Montpellier – EPHE – 1919 route de Mende, FR-34293 Montpellier Cedex 5, France. – B. Boyle and B. J. Enquist, Dept of Ecology and Evolutionary Biology, Univ. of Arizona, Tucson, AZ 85721, USA. – N. J. B. Kraft, Dept of Biology, Univ. of Maryland, College Park, MD 20742, USA. BJE also at: The Santa Fe inst., 1399 Hyde Park Road, Santa Fe, NM 87501, USA. – R. K. Peet, Dept of Biology, Univ. of North Carolina, Chapel Hill, NC 27599-3280, USA. -

The Asymmetry in the Great American Biotic Interchange in Mammals Is Consistent with Differential Susceptibility to Mammalian Predation
Coversheet This is the accepted manuscript (post-print version) of the article. Contentwise, the accepted manuscript version is identical to the final published version, but there may be differences in typography and layout. How to cite this publication Please cite the final published version: Faurby, S. and Svenning, J.-C. (2016), The asymmetry in the Great American Biotic Interchange in mammals is consistent with differential susceptibility to mammalian predation. Global Ecol. Biogeogr., 25: 1443–1453. doi:10.1111/geb.12504 Publication metadata Title: The asymmetry in the Great American Biotic Interchange in mammals is consistent with differential susceptibility to mammalian predation Author(s): Faurby, S. and Svenning, J.-C. Journal: Global Ecology and Biogeography DOI/Link: https://doi.org/10.1111/geb.12504 Document version: Accepted manuscript (post-print) This is the peer reviewed version of the following article: [Faurby, S. and Svenning, J.-C. (2016), The asymmetry in the Great American Biotic Interchange in mammals is consistent with differential susceptibility to mammalian predation. Global Ecol. Biogeogr., 25: 1443–1453. doi:10.1111/geb.12504], which has been published in final form at [https://doi.org/10.1111/geb.12504]. This article may be used for non-commercial purposes in accordance with Wiley Terms and Conditions for Self-Archiving. General Rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognize and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from the public portal for the purpose of private study or research. -

Charismatic Megafauna
KIDS CORNER CHARISMATIC MEGAFAUNA This document aims to teach you about megafauna. This presentation has the following structure: Slide 1 - What Are Megafauna? Slide 2 - Charismatic Megafauna Slide 3 - Megafauna Extinction Theories Slide 4 - Timeline Slide 5 - Living Megafauna Slide 6 - Extinct Australian Megafauna Slide 7 - Extinct African Megafauna Slide 8 - Case Study: Diprotodon optatum Slide 9 - Australian Curriculum Mapping KIDS CORNER CHARISMATIC MEGAFAUNA What Are Megafauna? Combining the Latin words for “large” (mega) and “animals” (fauna) creates the word “megafauna.” Megafauna are the largest animals on Earth – the ones that dominate the landscape during the time in which they live. Dinosaurs were certainly the megafauna of their time. And after the dinosaurs all died off in the mass extinction at the end of the Cretaceous period, new megafauna arose. They looked a lot like their modern descendants but were much bigger. Imagine wombats the size of a compact car, birds that stood taller than a human being, or snakes that make modern pythons look puny. Scientists consider animals that weigh more than 44 kilograms as adults to be megafauna. The term applies not only to mammals, but also to birds, reptiles, and amphibians—in short, all vertebrates, or animals with a backbone. By that definition, there are plenty of megafauna walking the Earth and swimming in its oceans today. Gorillas, elephants, and whales are prime examples. KIDS CORNER CHARISMATIC MEGAFAUNA Charismatic Megafauna The word “charismatic” means “charming” or “fascinating.” Conservationists coined the term “charismatic megafauna” during the 1980s to acknowledge that people find large animals very interesting, especially large animals that exhibit endearing or intriguing behaviour. -
Endangered Species
Not logged in Talk Contributions Create account Log in Article Talk Read Edit View history Endangered species From Wikipedia, the free encyclopedia Main page Contents For other uses, see Endangered species (disambiguation). Featured content "Endangered" redirects here. For other uses, see Endangered (disambiguation). Current events An endangered species is a species which has been categorized as likely to become Random article Conservation status extinct . Endangered (EN), as categorized by the International Union for Conservation of Donate to Wikipedia by IUCN Red List category Wikipedia store Nature (IUCN) Red List, is the second most severe conservation status for wild populations in the IUCN's schema after Critically Endangered (CR). Interaction In 2012, the IUCN Red List featured 3079 animal and 2655 plant species as endangered (EN) Help worldwide.[1] The figures for 1998 were, respectively, 1102 and 1197. About Wikipedia Community portal Many nations have laws that protect conservation-reliant species: for example, forbidding Recent changes hunting , restricting land development or creating preserves. Population numbers, trends and Contact page species' conservation status can be found in the lists of organisms by population. Tools Extinct Contents [hide] What links here Extinct (EX) (list) 1 Conservation status Related changes Extinct in the Wild (EW) (list) 2 IUCN Red List Upload file [7] Threatened Special pages 2.1 Criteria for 'Endangered (EN)' Critically Endangered (CR) (list) Permanent link 3 Endangered species in the United -

Ostrich Production Systems Part I: a Review
11111111111,- 1SSN 0254-6019 Ostrich production systems Food and Agriculture Organization of 111160mmi the United Natiorp str. ro ucti s ct1rns Part A review by Dr M.M. ,,hanawany International Consultant Part II Case studies by Dr John Dingle FAO Visiting Scientist Food and , Agriculture Organization of the ' United , Nations Ot,i1 The designations employed and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. M-21 ISBN 92-5-104300-0 Reproduction of this publication for educational or other non-commercial purposes is authorized without any prior written permission from the copyright holders provided the source is fully acknowledged. Reproduction of this publication for resale or other commercial purposes is prohibited without written permission of the copyright holders. Applications for such permission, with a statement of the purpose and extent of the reproduction, should be addressed to the Director, Information Division, Food and Agriculture Organization of the United Nations, Viale dells Terme di Caracalla, 00100 Rome, Italy. C) FAO 1999 Contents PART I - PRODUCTION SYSTEMS INTRODUCTION Chapter 1 ORIGIN AND EVOLUTION OF THE OSTRICH 5 Classification of the ostrich in the animal kingdom 5 Geographical distribution of ratites 8 Ostrich subspecies 10 The North -

The Impact of Climate Change on a Tropical Carnivore: from Individual to Species
The Impact of Climate Change on a Tropical Carnivore: From Individual to Species Daniella Dakin Rabaiotti A dissertation submitted for the degree of Doctor of Philosophy UCL 2 Declaration I, Daniella Rabaiotti, confirm the work presented in this thesis is my own. The research was supported by NERC through the London NERC DTP. All data analysis data visualisation and modelling was done by Daniella Rabaiotti. Tim Coulson provided training in individual based modelling. Mike Croucher assisted in code optimisation for the individual based model. All chapters of this thesis were written by Daniella Rabaiotti, with guidance and comments from Rosie Woodroffe and Richard Pearson. Tim Coulson provided comments on Chapter 4, and Rosemary Groom, J.W. McNutt and Jessica Watermeyer provided comments on Chapter 3. Data from Laikipia, Kenya, on wild dog survival and movements were collected by Rosie Woodroffe and the Kenya Rangelands Wild Dog and Cheetah Project. Data on wild dog mortality in Savé Valley, Zimbabwe were collected by Rosemary Groom and the Savé Valley team at the African Wildlife Conservation Fund. Data on wild dog mortality in the Okavango, Botswana, were collected by J.W. McNutt and the Botswana Predator Conservation Trust. Data on wild dog distributions were provided by the Rangewide Conservation Programme for Cheetah and African Wild Dogs. Cover art was designed by Daniella Rabaiotti and created by Selina Betts. Graphical abstracts were designed by Daniella Rabaiotti and Gaius J. Augustus and created by Gaius J. Augustus. 3 4 Abstract Climate change is impacting species globally. Predicting which species will be impacted, where, when, and by how much, is vital to conserve biodiversity in a warming world. -

What Are “Charismatic Species” ? F
1 / 8 What are “charismatic species” ? F. Ducarme. What are “charismatic species” for conservation biologists? Frédéric Ducarme, Gloria M. Luque, Franck Courchamp. Master BioSciences, Département de Biologie, Ecole Normale Supérieure de Lyon. Laboratoire Ecologie, Systématique & Evolution, CNRS,Université Paris XI. 2012-10-01 Keywords : Charismatic species, ecosystem management, adaptive management, endangered species, flagship species, indicator species, keystone species, umbrella species, culture, marketing. Current conservation biology often appeals to abstract concepts and models in order to embrace a wide reality with practical methods. Among these tools, there are different types of “focal” (or “surrogate”) species, like flagships, keystones, umbrellas or indicators, which all stand for a wider portion of biodiversity for different usages. These benchmarks are sometimes accused of being only “buzzwords” with few actual meaning or relevance, and are the subject of intense debate among conservationists. One of these terms, “charismatic species”, seems less debated, while it is widely used and does not seem to bear an obvious meaning. The purpose of this article is to draw a portrait of the use of this term in the conservation literature, and to sum up the debates it provokes and the problems it poses. We highlight that even if the actual signification of this concept lacks a consensus definition, the reality it indicates does exist and may have an underrated importance in biodiversity conservation. Introduction concept of charisma in conservation biology, and highlight its many meanings and controversial points. Conservation biology is usually defined as a holistic science [1], but biodiversity management often has to call for practical objectives. Conservation history shows Defining charisma and its role that it is impossible to measure or monitor all The term charisma sounds odd in such a research biodiversity, and even more when considering its state domain, as it has been borrowed from Latin and dynamics, so ecologists need proxies [2]. -

Variable Impact of Late-Quaternary Megafaunal Extinction in Causing
Variable impact of late-Quaternary megafaunal SPECIAL FEATURE extinction in causing ecological state shifts in North and South America Anthony D. Barnoskya,b,c,1, Emily L. Lindseya,b, Natalia A. Villavicencioa,b, Enrique Bostelmannd,2, Elizabeth A. Hadlye, James Wanketf, and Charles R. Marshalla,b aDepartment of Integrative Biology, University of California, Berkeley, CA 94720; bMuseum of Paleontology, University of California, Berkeley, CA 94720; cMuseum of Vertebrate Zoology, University of California, Berkeley, CA 94720; dRed Paleontológica U-Chile, Laboratoria de Ontogenia, Departamento de Biología, Facultad de Ciencias, Universidad de Chile, Chile; eDepartment of Biology, Stanford University, Stanford, CA 94305; and fDepartment of Geography, California State University, Sacramento, CA 95819 Edited by John W. Terborgh, Duke University, Durham, NC, and approved August 5, 2015 (received for review March 16, 2015) Loss of megafauna, an aspect of defaunation, can precipitate many megafauna loss, and if so, what does this loss imply for the future ecological changes over short time scales. We examine whether of ecosystems at risk for losing their megafauna today? megafauna loss can also explain features of lasting ecological state shifts that occurred as the Pleistocene gave way to the Holocene. We Approach compare ecological impacts of late-Quaternary megafauna extinction The late-Quaternary impact of losing 70–80% of the megafauna in five American regions: southwestern Patagonia, the Pampas, genera in the Americas (19) would be expected to trigger biotic northeastern United States, northwestern United States, and Berin- transitions that would be recognizable in the fossil record in at gia. We find that major ecological state shifts were consistent with least two respects. -

EARTH Ltd PME Threatened Habitats Handout
501-C-3 at Southwick’s Zoo, 2 Southwick Street, Mendon, MA 01756 PROTECTING MY EARTH: LOCALLY THREATENED HABITATS (MA) FACTS & FIGURES “Protecting My EARTH” is an environmental education program offered by EARTH Ltd. to help students learn how to take better care of their community and their planet. KEY TERMS AND DEFINITIONS • Conservation: a careful preservation and protection of something; especially planned management of a natural resource to prevent exploitation, destruction, or neglect • Habitat: the place or environment where a plant or animal naturally or normally lives and grows • Ecosystem: everything that exists in a particular environment • Endangered: a species in danger of becoming extinct • Extinct: no longer existing • Threatened: having an uncertain chance of continued survival; likely to become an endangered species • Vulnerable: easily damaged; likely to become an endangered species • CITES - Convention on International Trade of Endangered Species of Wild Fauna and Flora: an international agreement between governments effective since 1975. Its aim is to ensure that international trade in specimens of wild animals and plants does not threaten their survival. Roughly 5,600 species of animals and 30,000 species of plants are protected by CITES as of 2013. There are currently 181 countries (of about 196) that are contracting parties. • IUCN - International Union for the Conservation of Nature: world’s oldest and largest global environmental organization, with almost 1,300 government and NGO Members and more than 15,000 volunteer experts in 185 countries. Their work focuses on valuing and conserving nature, ensuring effective and equitable governance of its use, and deploying nature-based solutions to global challenges in climate, food and development. -

EFFECTS of PRE-SLAUGHTER HANDLING, TRANSPORTATION, and NUTRIENT SUPPLEMENTATION on OSTRICH WELFARE and PRODUCT QUALITY by Masoum
EFFECTS OF PRE-SLAUGHTER HANDLING, TRANSPORTATION, AND NUTRIENT SUPPLEMENTATION ON OSTRICH WELFARE AND PRODUCT QUALITY by Masoumeh Bejaei B.Sc. University of Tabriz, 2001 M.Sc. University of Tehran, 2004 M.Sc. The University of British Columbia, 2009 A THESIS SUBMITTED IN PARTIAL FULFILMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY in THE FACULTY OF GRADUATE STUDIES (Animal Science) THE UNIVERSITY OF BRITISH COLUMBIA (Vancouver) July 2013 © Masoumeh Bejaei, 2013 Abstract Ostriches (Struthio camelus) are the largest living birds with only two toes on each of two long feet that support a heavy body mass. This special anatomical feature creates problems for transporting ostriches. However, little research has been done to examine ostrich welfare during handling and transportation and how this relates to product quality. The main goal of this dissertation research was to find ways of improving ostrich welfare during pre-slaughter handling and transport, which would also contribute to increased product quality and decreased product losses. To achieve this goal, three related research projects were conducted. For the first research project, a producer survey was conducted in Canada and USA. From the survey results, I identified current ostrich pre-slaughter handling and transport norms (e.g., long transportation), and also potential welfare issues in the current ostrich pre-slaughter transport practices. Based on the identified potential welfare issues from the survey, an experiment (with 24 birds) was conducted to study effects of pre-transport handling on stress responses of ostriches. The results showed that the pre-transport handling process is stressful for ostriches and should be minimized.