The Bacterial Ectosymbionts of the Ciliate
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Morphology, Phylogeny, and Diversity of Trichonympha (Parabasalia: Hypermastigida) of the Wood-Feeding Cockroach Cryptocercus Punctulatus
J. Eukaryot. Microbiol., 56(4), 2009 pp. 305–313 r 2009 The Author(s) Journal compilation r 2009 by the International Society of Protistologists DOI: 10.1111/j.1550-7408.2009.00406.x Morphology, Phylogeny, and Diversity of Trichonympha (Parabasalia: Hypermastigida) of the Wood-Feeding Cockroach Cryptocercus punctulatus KEVIN J. CARPENTER, LAWRENCE CHOW and PATRICK J. KEELING Canadian Institute for Advanced Research, Botany Department, University of British Columbia, University Boulevard, Vancouver, BC, Canada V6T 1Z4 ABSTRACT. Trichonympha is one of the most complex and visually striking of the hypermastigote parabasalids—a group of anaerobic flagellates found exclusively in hindguts of lower termites and the wood-feeding cockroach Cryptocercus—but it is one of only two genera common to both groups of insects. We investigated Trichonympha of Cryptocercus using light and electron microscopy (scanning and transmission), as well as molecular phylogeny, to gain a better understanding of its morphology, diversity, and evolution. Microscopy reveals numerous new features, such as previously undetected bacterial surface symbionts, adhesion of post-rostral flagella, and a dis- tinctive frilled operculum. We also sequenced small subunit rRNA gene from manually isolated species, and carried out an environmental polymerase chain reaction (PCR) survey of Trichonympha diversity, all of which strongly supports monophyly of Trichonympha from Cryptocercus to the exclusion of those sampled from termites. Bayesian and distance methods support a relationship between Tricho- nympha species from termites and Cryptocercus, although likelihood analysis allies the latter with Eucomonymphidae. A monophyletic Trichonympha is of great interest because recent evidence supports a sister relationship between Cryptocercus and termites, suggesting Trichonympha predates the Cryptocercus-termite divergence. -

Culturing and Targeted Pacbio RS Amplicon Sequencing Reveals a Higher Order Taxonomic Diversity and Global Distribution
bioRxiv preprint doi: https://doi.org/10.1101/199125; this version posted October 8, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. Enigmatic Diphyllatea eukaryotes: Culturing and targeted PacBio RS amplicon sequencing reveals a higher order taxonomic diversity and global distribution Orr Russell J.S.1,2*, Zhao Sen3,4, Klaveness Dag5, Yabuki Akinori6, Ikeda Keiji7, Makoto M. Watanabe7, Shalchian-Tabrizi Kamran1,2* 1 Section for Genetics and Evolutionary Biology (EVOGENE), Department of Biosciences, University of Oslo, Oslo, Norway 2 Centre for Integrative Microbial Evolution (CIME), Section for Genetics and Evolutionary Biology (EVOGENE), Department of Biosciences, University of Oslo, Oslo, Norway 3 Department of Molecular Oncology, Institute of Cancer Research, Oslo University Hospital-Radiumhospitalet, Oslo, Norway 4 Medical Faculty, Center for Cancer Biomedicine, University of Oslo University Hospital, Oslo, Norway 5 Section for Aquatic Biology and Toxicology (AQUA), Department of Biosciences, University of Oslo, Oslo, Norway 6 Japan Agency for Marine-Earth Sciences and Technology (JAMSTEC), 2-15 Natsushima, Yokosuka, Kanagawa 237-0061, Japan 7 Faculty of Life and Environmental Sciences, University of Tsukuba, 1-1-1 Tennodai, Tsukuba, Ibaraki 305-8572, Japan * Corresponding authors: Russell J. S. Orr & Kamran Shalchian-Tabrizi Email: [email protected] Mobile: +4748187013 Email: [email protected] Mobile: +4741045328 Address: Kristine Bonnevies hus, Blindernveien 31, 0371 Oslo, Norway Keywords: Diphyllatea, PacBio, rRNA, phylogeny, diversity, Collodictyon, amplicon, Sulcozoa 1 bioRxiv preprint doi: https://doi.org/10.1101/199125; this version posted October 8, 2017. -

Biology of Protists Video and DVD Guide. the BIOLOGY of PROTISTS Produced by Biomedia ASSOCIATES ©2003 -- Running Time 20 Minutes
Biology of Protists video and DVD guide. THE BIOLOGY OF PROTISTS Produced by BioMEDIA ASSOCIATES ©2003 -- Running time 20 minutes. Order Toll Free (877) 661-5355 or FAX (843) 470-0237 Or mail orders to eBioMEDIA, P.O. Box 1234, Beaufort, SC 29901–1234 (IMAGES IN THIS GUIDE ARE FROM THE VIDEO PROGRAM) Cosmerium, a green alga Arcella, a shelled ameoba The goal of this program is to show a representative sample of the great diversity of protists, and to show why they need a new classification reflecting our growing understanding of their long evolutionary history. The protists shown can be found in habitats such as: roadside puddles, park duck ponds, aquariums, birdbaths and in the gut of termites. We hope that these observations will encourage students to collect pond water samples and see for themselves this amazing hidden world. The live photography was accomplished using a variety microscope techniques, including dark field (good for showing the natural color of subjects) and the most advanced forms of differential interference contrast (DIC gives contrast to transparent structures that would be invisible in normal bright field microscopy). While these forms of imaging living protists are particularly revealing for some aspects of micro-organism biology, all of the organisms seen here can be studied successfully with student microscopes. IMPORTANT NOTE: This program packs a lot of information and a wealth of observational data into nine different modules. We recommend that the material be viewed module by module, stopping for discussion and replay as needed. “Discussion starters” for the various modules are provided in this guide. -

University of Oklahoma
UNIVERSITY OF OKLAHOMA GRADUATE COLLEGE MACRONUTRIENTS SHAPE MICROBIAL COMMUNITIES, GENE EXPRESSION AND PROTEIN EVOLUTION A DISSERTATION SUBMITTED TO THE GRADUATE FACULTY in partial fulfillment of the requirements for the Degree of DOCTOR OF PHILOSOPHY By JOSHUA THOMAS COOPER Norman, Oklahoma 2017 MACRONUTRIENTS SHAPE MICROBIAL COMMUNITIES, GENE EXPRESSION AND PROTEIN EVOLUTION A DISSERTATION APPROVED FOR THE DEPARTMENT OF MICROBIOLOGY AND PLANT BIOLOGY BY ______________________________ Dr. Boris Wawrik, Chair ______________________________ Dr. J. Phil Gibson ______________________________ Dr. Anne K. Dunn ______________________________ Dr. John Paul Masly ______________________________ Dr. K. David Hambright ii © Copyright by JOSHUA THOMAS COOPER 2017 All Rights Reserved. iii Acknowledgments I would like to thank my two advisors Dr. Boris Wawrik and Dr. J. Phil Gibson for helping me become a better scientist and better educator. I would also like to thank my committee members Dr. Anne K. Dunn, Dr. K. David Hambright, and Dr. J.P. Masly for providing valuable inputs that lead me to carefully consider my research questions. I would also like to thank Dr. J.P. Masly for the opportunity to coauthor a book chapter on the speciation of diatoms. It is still such a privilege that you believed in me and my crazy diatom ideas to form a concise chapter in addition to learn your style of writing has been a benefit to my professional development. I’m also thankful for my first undergraduate research mentor, Dr. Miriam Steinitz-Kannan, now retired from Northern Kentucky University, who was the first to show the amazing wonders of pond scum. Who knew that studying diatoms and algae as an undergraduate would lead me all the way to a Ph.D. -

ABSTRACT Gregarine Parasitism in Dragonfly Populations of Central
ABSTRACT Gregarine Parasitism in Dragonfly Populations of Central Texas with an Assessment of Fitness Costs in Erythemis simplicicollis Jason L. Locklin, Ph.D. Mentor: Darrell S. Vodopich, Ph.D. Dragonfly parasites are widespread and frequently include gregarines (Phylum Apicomplexa) in the gut of the host. Gregarines are ubiquitous protozoan parasites that infect arthropods worldwide. More than 1,600 gregarine species have been described, but only a small percentage of invertebrates have been surveyed for these apicomplexan parasites. Some consider gregarines rather harmless, but recent studies suggest otherwise. Odonate-gregarine studies have more commonly involved damselflies, and some have considered gregarines to rarely infect dragonflies. In this study, dragonfly populations were surveyed for gregarines and an assessment of fitness costs was made in a common and widespread host species, Erythemis simplicicollis. Adult dragonfly populations were surveyed weekly at two reservoirs in close proximity to one another and at a flow-through wetland system. Gregarine prevalences and intensities were compared within host populations between genders, among locations, among wing loads, and through time. Host fitness parameters measured included wing load, egg size, clutch size, and total egg count. Of the 37 dragonfly species surveyed, 14 species (38%) hosted gregarines. Thirteen of those species were previously unreported as hosts. Gregarine prevalences ranged from 2% – 52%. Intensities ranged from 1 – 201. Parasites were aggregated among their hosts. Gregarines were found only in individuals exceeding a minimum wing load, indicating that gregarines are likely not transferred from the naiad to adult during emergence. Prevalence and intensity exhibited strong seasonality during both years at one of the reservoirs, but no seasonal trend was detected at the wetland. -

Aquatic Microbial Ecology 62:139–152 (2011)
The following supplement accompanies the article Airborne microeukaryote colonists in experimental water containers: diversity, succession, life histories and established food webs Savvas Genitsaris1, Maria Moustaka-Gouni1,*, Konstantinos A. Kormas2 1Department of Botany, School of Biology, Aristotle University of Thessaloniki, 541 24 Thessaloniki, Greece 2Department of Ichthyology and Aquatic Environment, School of Agricultural Sciences, University of Thessaly, 384 46 Nea Ionia, Magnisia, Greece *Corresponding author. Email: [email protected] Aquatic Microbial Ecology 62:139–152 (2011) Supplement. Additional data Fig. S1. Clone library coverage based on Good’s C estimator of the eukaryotic 18S rDNA clone libraries from the water containers. The ratio observed phylotypes: predicted phylotypes (SChao1) was 0.7 in autumn, 0.87 in winter and 0.47 in spring. 2 Fig. S2. Phylogenetic tree of relationships of 18S rDNA (ca. 1600 bp) of the representative unique (grouped on ≥98% similarity) eukaryotic clones (in bold) found in the tap water containers, based on the neighbour-joining method as determined by distance Jukes–Cantor analysis. One thousand bootstrap analyses (distance) were conducted. GenBank numbers are shown in parentheses. Scale bar represents 2% estimated. 3 Table S1. Daily meteorological data in the city of Thessaloniki during the sampling periods of the study Air temperature (oC) Rainfall Sunshine RH Wind speed (mm) (min) (%) (m s-1) min max mean min max mean min max mean min max mean min max mean Autumn 2007 7.1 17.1 11.9 0 18.3 1.9 0 494.5 203.4 33.2 90.5 70.7 0.9 5.5 2.0 Winter 2007–8 –0.7 13.5 7.6 0 17.8 0.7 0 555.7 277.6 24.5 88.7 63.4 0.8 7.7 2.1 Spring 2008 8.5 16.9 13.0 0 34.7 1.9 0 663.7 363.7 36.8 91.0 66.8 1.2 3.6 1.8 Table S2. -

Multigene Eukaryote Phylogeny Reveals the Likely Protozoan Ancestors of Opis- Thokonts (Animals, Fungi, Choanozoans) and Amoebozoa
Accepted Manuscript Multigene eukaryote phylogeny reveals the likely protozoan ancestors of opis- thokonts (animals, fungi, choanozoans) and Amoebozoa Thomas Cavalier-Smith, Ema E. Chao, Elizabeth A. Snell, Cédric Berney, Anna Maria Fiore-Donno, Rhodri Lewis PII: S1055-7903(14)00279-6 DOI: http://dx.doi.org/10.1016/j.ympev.2014.08.012 Reference: YMPEV 4996 To appear in: Molecular Phylogenetics and Evolution Received Date: 24 January 2014 Revised Date: 2 August 2014 Accepted Date: 11 August 2014 Please cite this article as: Cavalier-Smith, T., Chao, E.E., Snell, E.A., Berney, C., Fiore-Donno, A.M., Lewis, R., Multigene eukaryote phylogeny reveals the likely protozoan ancestors of opisthokonts (animals, fungi, choanozoans) and Amoebozoa, Molecular Phylogenetics and Evolution (2014), doi: http://dx.doi.org/10.1016/ j.ympev.2014.08.012 This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain. 1 1 Multigene eukaryote phylogeny reveals the likely protozoan ancestors of opisthokonts 2 (animals, fungi, choanozoans) and Amoebozoa 3 4 Thomas Cavalier-Smith1, Ema E. Chao1, Elizabeth A. Snell1, Cédric Berney1,2, Anna Maria 5 Fiore-Donno1,3, and Rhodri Lewis1 6 7 1Department of Zoology, University of Oxford, South Parks Road, Oxford OX1 3PS, UK. -
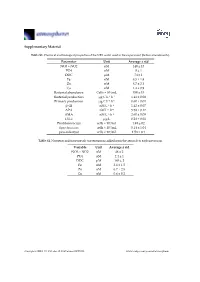
Supplementary Material Parameter Unit Average ± Std NO3 + NO2 Nm
Supplementary Material Table S1. Chemical and biological properties of the NRS water used in the experiment (before amendments). Parameter Unit Average ± std NO3 + NO2 nM 140 ± 13 PO4 nM 8 ± 1 DOC μM 74 ± 1 Fe nM 8.5 ± 1.8 Zn nM 8.7 ± 2.1 Cu nM 1.4 ± 0.9 Bacterial abundance Cells × 104/mL 350 ± 15 Bacterial production μg C L−1 h−1 1.41 ± 0.08 Primary production μg C L−1 h−1 0.60 ± 0.01 β-Gl nM L−1 h−1 1.42 ± 0.07 APA nM L−1 h−1 5.58 ± 0.17 AMA nM L−1·h−1 2.60 ± 0.09 Chl-a μg/L 0.28 ± 0.01 Prochlorococcus cells × 104/mL 1.49 ± 02 Synechococcus cells × 104/mL 5.14 ± 1.04 pico-eukaryot cells × 103/mL 1.58 × 0.1 Table S2. Nutrients and trace metals concentrations added from the aerosols to each mesocosm. Variable Unit Average ± std NO3 + NO2 nM 48 ± 2 PO4 nM 2.4 ± 1 DOC μM 165 ± 2 Fe nM 2.6 ± 1.5 Zn nM 6.7 ± 2.5 Cu nM 0.6 ± 0.2 Atmosphere 2019, 10, 358; doi:10.3390/atmos10070358 www.mdpi.com/journal/atmosphere Atmosphere 2019, 10, 358 2 of 6 Table S3. ANOVA test results between control, ‘UV-treated’ and ‘live-dust’ treatments at 20 h or 44 h, with significantly different values shown in bold. ANOVA df Sum Sq Mean Sq F Value p-value Chl-a 20 H 2, 6 0.03, 0.02 0.02, 0 4.52 0.0634 44 H 2, 6 0.02, 0 0.01, 0 23.13 0.002 Synechococcus Abundance 20 H 2, 7 8.23 × 107, 4.11 × 107 4.11 × 107, 4.51 × 107 0.91 0.4509 44 H 2, 7 5.31 × 108, 6.97 × 107 2.65 × 108, 1.16 × 107 22.84 0.0016 Prochlorococcus Abundance 20 H 2, 8 4.22 × 107, 2.11 × 107 2.11 × 107, 2.71 × 106 7.77 0.0216 44 H 2, 8 9.02 × 107, 1.47 × 107 4.51 × 107, 2.45 × 106 18.38 0.0028 Pico-eukaryote -

Phylogenomic Analysis of Balantidium Ctenopharyngodoni (Ciliophora, Litostomatea) Based on Single-Cell Transcriptome Sequencing
Parasite 24, 43 (2017) © Z. Sun et al., published by EDP Sciences, 2017 https://doi.org/10.1051/parasite/2017043 Available online at: www.parasite-journal.org RESEARCH ARTICLE Phylogenomic analysis of Balantidium ctenopharyngodoni (Ciliophora, Litostomatea) based on single-cell transcriptome sequencing Zongyi Sun1, Chuanqi Jiang2, Jinmei Feng3, Wentao Yang2, Ming Li1,2,*, and Wei Miao2,* 1 Hubei Key Laboratory of Animal Nutrition and Feed Science, Wuhan Polytechnic University, Wuhan 430023, PR China 2 Institute of Hydrobiology, Chinese Academy of Sciences, No. 7 Donghu South Road, Wuchang District, Wuhan 430072, Hubei Province, PR China 3 Department of Pathogenic Biology, School of Medicine, Jianghan University, Wuhan 430056, PR China Received 22 April 2017, Accepted 12 October 2017, Published online 14 November 2017 Abstract- - In this paper, we present transcriptome data for Balantidium ctenopharyngodoni Chen, 1955 collected from the hindgut of grass carp (Ctenopharyngodon idella). We evaluated sequence quality and de novo assembled a preliminary transcriptome, including 43.3 megabits and 119,141 transcripts. Then we obtained a final transcriptome, including 17.7 megabits and 35,560 transcripts, by removing contaminative and redundant sequences. Phylogenomic analysis based on a supermatrix with 132 genes comprising 53,873 amino acid residues and phylogenetic analysis based on SSU rDNA of 27 species were carried out herein to reveal the evolutionary relationships among six ciliate groups: Colpodea, Oligohymenophorea, Litostomatea, Spirotrichea, Hetero- trichea and Protocruziida. The topologies of both phylogenomic and phylogenetic trees are discussed in this paper. In addition, our results suggest that single-cell sequencing is a sound method of obtaining sufficient omics data for phylogenomic analysis, which is a good choice for uncultivable ciliates. -

Announcements Protists - Outline Reading: Chap
Announcements Protists - Outline Reading: Chap. 29 • Relevant reading BEFORE lab this week: Ch. 31 I. Introduction • Bring lab atlas AND textbook to lab. A. Diversity of life styles IV. Evolutionary history • Extra credit opportunity: B. Functional classifications A. Kingdom Protista? – Salmon Summit: Wed. 11/3/10, 8-4:45 pm II. Ecological importance B. How are they related St. Luke’s Community Health Education Center to each other? A. Algae C. How did they arise? Bellingham, WA (checking on registration) B. Protozoans D. How are they related III. Life cycles to plants? A. The three basic types B. Examples Diatom I.A. Diversity of life styles Size 1. Size 10 μm 2. Morphology 3. Motility Kelp 4. Energy sources 6 orders of magnitude! 60 m Filamentous (Golden algae) Morphology Gradient in complexity Unicellular (Euglena) Colonial (Pandorina) Multicellular (kelp) 1 Crawling (pseudopodia) Cell walls – protection & support Planktonic Amoeba Diatoms Cilia Flagella Motility Fastened Amoeba Paramecium Euglena Kelp No cell wall Energy source - photoautotrophs Variation in photosynthetic pigments Energy source - heterotrophs Ingestive feeders Absorptive feeders: decomp., parasites I.B. Functional classifications Particle feeder (Stentor) Protozoans - “animal like” Parasite Algae - “plant-like”, i.e., photosynthetic (Trypanosoma) - Eukaryotic photosynthetic organisms that are not plants Decomposer Mix - simple to bizarre (slime mold Don’t necessarily relate to taxonomic Physarum) (ingestive) relationships and evolutionary history Predator (Amoeba) 2 Cellular slime mold – unicellular or multicellular? Mixotroph example - Euglena Cells (n) aggregate when food is scarce Amoebae (n) Spores germinate Amoebae (n) germinate (n) from zygote from spores SEXUAL ASEXUAL REPRODUCTION REPRODUCTION Fruiting body Stalk Giant cell Migrating individual (2n) (slug) (n) Two cells (n) in aggregation fuse, then consume other cells Fig. -

Protist Phylogeny and the High-Level Classification of Protozoa
Europ. J. Protistol. 39, 338–348 (2003) © Urban & Fischer Verlag http://www.urbanfischer.de/journals/ejp Protist phylogeny and the high-level classification of Protozoa Thomas Cavalier-Smith Department of Zoology, University of Oxford, South Parks Road, Oxford, OX1 3PS, UK; E-mail: [email protected] Received 1 September 2003; 29 September 2003. Accepted: 29 September 2003 Protist large-scale phylogeny is briefly reviewed and a revised higher classification of the kingdom Pro- tozoa into 11 phyla presented. Complementary gene fusions reveal a fundamental bifurcation among eu- karyotes between two major clades: the ancestrally uniciliate (often unicentriolar) unikonts and the an- cestrally biciliate bikonts, which undergo ciliary transformation by converting a younger anterior cilium into a dissimilar older posterior cilium. Unikonts comprise the ancestrally unikont protozoan phylum Amoebozoa and the opisthokonts (kingdom Animalia, phylum Choanozoa, their sisters or ancestors; and kingdom Fungi). They share a derived triple-gene fusion, absent from bikonts. Bikonts contrastingly share a derived gene fusion between dihydrofolate reductase and thymidylate synthase and include plants and all other protists, comprising the protozoan infrakingdoms Rhizaria [phyla Cercozoa and Re- taria (Radiozoa, Foraminifera)] and Excavata (phyla Loukozoa, Metamonada, Euglenozoa, Percolozoa), plus the kingdom Plantae [Viridaeplantae, Rhodophyta (sisters); Glaucophyta], the chromalveolate clade, and the protozoan phylum Apusozoa (Thecomonadea, Diphylleida). Chromalveolates comprise kingdom Chromista (Cryptista, Heterokonta, Haptophyta) and the protozoan infrakingdom Alveolata [phyla Cilio- phora and Miozoa (= Protalveolata, Dinozoa, Apicomplexa)], which diverged from a common ancestor that enslaved a red alga and evolved novel plastid protein-targeting machinery via the host rough ER and the enslaved algal plasma membrane (periplastid membrane). -

Protistology an International Journal Vol
Protistology An International Journal Vol. 10, Number 2, 2016 ___________________________________________________________________________________ CONTENTS INTERNATIONAL SCIENTIFIC FORUM «PROTIST–2016» Yuri Mazei (Vice-Chairman) Welcome Address 2 Organizing Committee 3 Organizers and Sponsors 4 Abstracts 5 Author Index 94 Forum “PROTIST-2016” June 6–10, 2016 Moscow, Russia Website: http://onlinereg.ru/protist-2016 WELCOME ADDRESS Dear colleagues! Republic) entitled “Diplonemids – new kids on the block”. The third lecture will be given by Alexey The Forum “PROTIST–2016” aims at gathering Smirnov (Saint Petersburg State University, Russia): the researchers in all protistological fields, from “Phylogeny, diversity, and evolution of Amoebozoa: molecular biology to ecology, to stimulate cross- new findings and new problems”. Then Sandra disciplinary interactions and establish long-term Baldauf (Uppsala University, Sweden) will make a international scientific cooperation. The conference plenary presentation “The search for the eukaryote will cover a wide range of fundamental and applied root, now you see it now you don’t”, and the fifth topics in Protistology, with the major focus on plenary lecture “Protist-based methods for assessing evolution and phylogeny, taxonomy, systematics and marine water quality” will be made by Alan Warren DNA barcoding, genomics and molecular biology, (Natural History Museum, United Kingdom). cell biology, organismal biology, parasitology, diversity and biogeography, ecology of soil and There will be two symposia sponsored by ISoP: aquatic protists, bioindicators and palaeoecology. “Integrative co-evolution between mitochondria and their hosts” organized by Sergio A. Muñoz- The Forum is organized jointly by the International Gómez, Claudio H. Slamovits, and Andrew J. Society of Protistologists (ISoP), International Roger, and “Protists of Marine Sediments” orga- Society for Evolutionary Protistology (ISEP), nized by Jun Gong and Virginia Edgcomb.