The Visual Ecology of Speyeria Mormonia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Exploitation of Lycaenid-Ant Mutualisms by Braconid Parasitoids
31(3-4):153-168,Journal of Research 1992 on the Lepidoptera 31(3-4):153-168, 1992 153 Exploitation of lycaenid-ant mutualisms by braconid parasitoids Konrad Fiedler1, Peter Seufert1, Naomi E. Pierce2, John G. Pearson3 and Hans-Thomas Baumgarten1 1 Theodor-Boveri-Zentrum für Biowissenschaften, Lehrstuhl Zoologie II, Universität Würzburg, Am Hubland, D-97074 Würzburg, Germany 2 Museum of Comparative Zoology, Harvard University, 26 Oxford Street, Cambridge, MA 02138-2902, USA 3 Division of Natural Sciences and Mathematics, Western State College, Gunnison, CO 81230, USA Abstract. Larvae of 17 Lycaenidae butterfly species from Europe, North America, South East Asia and Australia were observed to retain at least some of their adaptations related to myrmecophily even after parasitic braconid larvae have emerged from them. The myrme- cophilous glandular organs and vibratory muscles of such larval carcasses remain functional for up to 8 days. The cuticle of lycaenid larvae contains extractable “adoption substances” which elicit anten- nal drumming in their tending ants. These adoption substances, as well, appear to persist in a functional state beyond parasitoid emer- gence, and the larval carcasses are hence tended much like healthy caterpillars. In all examples, the braconids may receive selective advantages through myrmecophily of their host larvae, instead of being suppressed by the ant guard. Interactions where parasitoids exploit the ant-mutualism of their lycaenid hosts have as yet been recorded only from the Apanteles group in the Braconidae- Microgasterinae. KEY WORDS: Lycaenidae, Formicidae, myrmecophily, adoption sub- stances, parasitoids, Braconidae, Apanteles, defensive mechanisms INTRODUCTION Parasitoid wasps or flies are major enemies of the early stages of most Lepidoptera (Shaw 1990, Weseloh 1993). -

Lepidoptera: Lycaenidae: Lipteninae) Uses a Color-Generating Mechanism Widely Applied by Butterflies
Journal of Insect Science, (2018) 18(3): 6; 1–8 doi: 10.1093/jisesa/iey046 Research The Only Blue Mimeresia (Lepidoptera: Lycaenidae: Lipteninae) Uses a Color-Generating Mechanism Widely Applied by Butterflies Zsolt Bálint,1,5 Szabolcs Sáfián,2 Adrian Hoskins,3 Krisztián Kertész,4 Antal Adolf Koós,4 Zsolt Endre Horváth,4 Gábor Piszter,4 and László Péter Biró4 1Hungarian Natural History Museum, Budapest, Hungary, 2Faculty of Forestry, University of West Hungary, Sopron, Hungary, 3Royal Entomological Society, London, United Kingdom, 4Institute of Technical Physics and Materials Science, Centre for Energy Research, Budapest, Hungary, and 5Corresponding author, e-mail: [email protected] Subject Editor: Konrad Fiedler Received 21 February 2018; Editorial decision 25 April 2018 Abstract The butterflyMimeresia neavei (Joicey & Talbot, 1921) is the only species in the exclusively African subtribal clade Mimacraeina (Lipteninae: Lycaenidae: Lepidoptera) having sexual dimorphism expressed by structurally blue- colored male and pigmentary colored orange–red female phenotypes. We investigated the optical mechanism generating the male blue color by various microscopic and experimental methods. It was found that the blue color is produced by the lower lamina of the scale acting as a thin film. This kind of color production is not rare in day-flying Lepidoptera, or in other insect orders. The biological role of the blue color of M. neavei is not yet well understood, as all the other species in the clade lack structural coloration, and have less pronounced sexual dimorphism, and are involved in mimicry-rings. Key words: Africa, Lycaenidae, mimicry, thin film, wing scale The late John Nevill Eliot in his fundamental work on Lycaenidae blue dorsal wing surface, whilst the female with its bright orange classification subdivided the family into sections, tribes, and sub- appearance is a typical mimeresine. -

Philmont Butterflies
PHILMONT AREA BUTTERFLIES Mexican Yellow (Eurema mexicana) Melissa Blue (Lycaeides melissa) Sleepy Orange (Eurema nicippe) Greenish Blue (Plebejus saepiolus) PAPILIONIDAE – Swallowtails Dainty Sulfur (Nathalis iole) Boisduval’s Blue (Icaricia icarioides) Subfamily Parnassiinae – Parnassians Lupine Blue (Icaricia lupini) Rocky Mountain Parnassian (Parnassius LYCAENIDAE – Gossamer-wings smintheus) Subfamily Lycaeninae – Coppers RIODINIDAE – Metalmarks Tailed Copper (Lycaena arota) Mormon Metalmark (Apodemia morma) Subfamily Papilioninae – Swallowtails American Copper (Lycaena phlaeas) Nais Metalmark (Apodemia nais) Black Swallowtail (Papilio polyxenes) Lustrous Copper (Lycaena cupreus) Old World Swallowtail (Papilio machaon) Bronze Copper (Lycaena hyllus) NYMPHALIDAE – Brush-footed Butterflies Anise Swallowtail (Papilio zelicaon) Ruddy Copper (Lycaena rubidus) Subfamily Libytheinae – Snout Butterflies Western Tiger Swallowtail (Papilio rutulus) Blue Copper (Lycaena heteronea) American Snout (Libytheana carinenta) Pale Swallowtail (Papilio eurymedon) Purplish Copper (Lycaena helloides) Two-tailed Swallowtail (Papilio multicaudatus) Subfamily Heliconiinae – Long-wings Subfamily Theclinae – Hairstreaks Gulf Fritillary (Agraulis vanillae) PIERIDAE – Whites & Sulfurs Colorado Hairstreak (Hypaurotis crysalus) Subfamily Pierinae – Whites Great Purple Hairstreak (Atlides halesus) Subfamily Argynninae – Fritillaries Pine White (Neophasia menapia) Southern Hairstreak (Fixsenia favonius) Variegated Fritillary (Euptoieta claudia) Becker’s White (Pontia -

Making Eggs from Nectar: the Role of Life History and Dietary Carbon Turnover in Butterfly Reproductive Resource Allocation
OIKOS 105: 279Á/291, 2004 Making eggs from nectar: the role of life history and dietary carbon turnover in butterfly reproductive resource allocation Diane M. O’Brien, Carol L. Boggs and Marilyn L. Fogel O’Brien, D. M., Boggs, C. L. and Fogel, M. L. 2004. Making eggs from nectar: the role of life history and dietary carbon turnover in butterfly reproductive resource allocation. Á/ Oikos 105: 279Á/291. The diets of many butterflies and moths change dramatically with development: from herbivory in the larvae to nectarivory in the adults. These diets are nutritionally distinct, and thus are likely to contribute differentially to egg manufacture. We examine the use of dietary resources in egg manufacture by four butterfly species with different patterns of oviposition and lifespan; three in the Nymphalidae (Euphydryas chalcedona, Speyeria mormonia and Heliconius charitonia), and one in the Pieridae (Colias eurytheme). Each species was fed two isotopically distinct adult diets based on sucrose, both of which differed from the larval hostplant in 13C content. Egg isotopic composition was analyzed to quantify the contribution of carbon from the larval and adult diets to egg manufacture. In all four species, egg 13C content increased to an asymptotic maximum with time, indicating that adult diet is an increasingly important source of egg carbon . The 13C increase closely resembled that of a nectar-feeding hawkmoth, and was well-described by a model of carbon flow proposed for that species. This similarity suggests that the turnover from larval to adult dietary support of egg manufacture is conserved among nectar-feeding Lepidoptera. Species varied widely in the maximum % egg carbon that derives from the adult diet, from 44% in E. -

Origins of Six Species of Butterflies Migrating Through Northeastern
diversity Article Origins of Six Species of Butterflies Migrating through Northeastern Mexico: New Insights from Stable Isotope (δ2H) Analyses and a Call for Documenting Butterfly Migrations Keith A. Hobson 1,2,*, Jackson W. Kusack 2 and Blanca X. Mora-Alvarez 2 1 Environment and Climate Change Canada, 11 Innovation Blvd., Saskatoon, SK S7N 0H3, Canada 2 Department of Biology, University of Western Ontario, Ontario, ON N6A 5B7, Canada; [email protected] (J.W.K.); [email protected] (B.X.M.-A.) * Correspondence: [email protected] Abstract: Determining migratory connectivity within and among diverse taxa is crucial to their conservation. Insect migrations involve millions of individuals and are often spectacular. However, in general, virtually nothing is known about their structure. With anthropogenically induced global change, we risk losing most of these migrations before they are even described. We used stable hydrogen isotope (δ2H) measurements of wings of seven species of butterflies (Libytheana carinenta, Danaus gilippus, Phoebis sennae, Asterocampa leilia, Euptoieta claudia, Euptoieta hegesia, and Zerene cesonia) salvaged as roadkill when migrating in fall through a narrow bottleneck in northeast Mexico. These data were used to depict the probabilistic origins in North America of six species, excluding the largely local E. hegesia. We determined evidence for long-distance migration in four species (L. carinenta, E. claudia, D. glippus, Z. cesonia) and present evidence for panmixia (Z. cesonia), chain (Libytheana Citation: Hobson, K.A.; Kusack, J.W.; Mora-Alvarez, B.X. Origins of Six carinenta), and leapfrog (Danaus gilippus) migrations in three species. Our investigation underlines Species of Butterflies Migrating the utility of the stable isotope approach to quickly establish migratory origins and connectivity in through Northeastern Mexico: New butterflies and other insect taxa, especially if they can be sampled at migratory bottlenecks. -

A Rearing Method for Argynnis (Speyeria) Diana
Hindawi Publishing Corporation Psyche Volume 2011, Article ID 940280, 6 pages doi:10.1155/2011/940280 Research Article ARearingMethodforArgynnis (Speyeria) diana (Lepidoptera: Nymphalidae) That Avoids Larval Diapause Carrie N. Wells, Lindsey Edwards, Russell Hawkins, Lindsey Smith, and David Tonkyn Department of Biological Sciences, Clemson University, 132 Long Hall, Clemson, SC 29634, USA Correspondence should be addressed to Carrie N. Wells, [email protected] Received 25 May 2011; Accepted 4 August 2011 Academic Editor: Russell Jurenka Copyright © 2011 Carrie N. Wells et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. We describe a rearing protocol that allowed us to raise the threatened butterfly, Argynnis diana (Nymphalidae), while bypassing the first instar overwintering diapause. We compared the survival of offspring reared under this protocol from field-collected A. diana females from North Carolina, Georgia, and Tennessee. Larvae were reared in the lab on three phylogenetically distinct species of Southern Appalachian violets (Viola sororia, V. pubescens,andV. pedata). We assessed larval survival in A. diana to the last instar, pupation, and adulthood. Males reared in captivity emerged significantly earlier than females. An ANOVA revealed no evidence of host plant preference by A. diana toward three native violet species. We suggest that restoration of A. diana habitat which promotes a wide array of larval and adult host plants, is urgently needed to conserve this imperiled species into the future. 1. Introduction larvae in cold storage blocks and storing them under con- trolled refrigerated conditions for the duration of their The Diana fritillary, Argynnis (Speyeria) diana (Cramer overwintering period [10]. -

Journal of the Lepidopterists' Society
J OURNAL OF T HE L EPIDOPTERISTS’ S OCIETY Volume 62 2008 Number 2 Journal of the Lepidopterists’ Society 61(2), 2007, 61–66 COMPARATIVE STUDIES ON THE IMMATURE STAGES AND DEVELOPMENTAL BIOLOGY OF FIVE ARGYNNIS SPP. (SUBGENUS SPEYERIA) (NYMPHALIDAE) FROM WASHINGTON DAVID G. JAMES Department of Entomology, Washington State University, Irrigated Agriculture Research and Extension Center, 24105 North Bunn Road, Prosser, Washington 99350; email: [email protected] ABSTRACT. Comparative illustrations and notes on morphology and biology are provided on the immature stages of five Arg- ynnis spp. (A. cybele leto, A. coronis simaetha, A. zerene picta, A. egleis mcdunnoughi, A. hydaspe rhodope) found in the Pacific Northwest. High quality images allowed separation of the five species in most of their immature stages. Sixth instars of all species possessed a fleshy, eversible osmeterium-like gland located ventrally between the head and first thoracic segment. Dormant first in- star larvae of all species exposed to summer-like conditions (25 ± 0.5º C and continuous illumination), 2.0–2.5 months after hatch- ing, did not feed and died within 6–9 days, indicating the larvae were in diapause. Overwintering of first instars for ~ 80 days in dark- ness at 5 ± 0.5º C, 75 ± 5% r.h. resulted in minimal mortality. Subsequent exposure to summer-like conditions (25 ± 0.5º C and continuous illumination) resulted in breaking of dormancy and commencement of feeding in all species within 2–5 days. Durations of individual instars and complete post-larval feeding development durations were similar for A. coronis, A. zerene, A. egleis and A. -
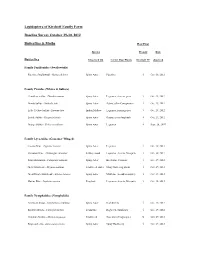
Butterflies and Moths List
Lepidoptera of Kirchoff Family Farm Baseline Survey October 29-30, 2012 Butterflies & Moths Host Plant Species Present Date Butterflies Observed On Larval Host Plants Kirchoff FF observed Family Papilionidae (Swallowtails) Pipevine Swallowtail - Battus philenor Spiny Aster Pipevine √ Oct. 30, 2012 Family Pieridae (Whites & Sulfurs) Cloudless Sulfur - Phoebis sennae Spiny Aster Legumes, clovers, peas √ Oct. 29, 2012 Dainty Sulfur - Nathalis iole Spiny Aster Asters, other Com;posites √ Oct. 29, 2012 Little Yellow Sulfur - Eurema lisa Indian Mallow Legumes, partridge pea √ Oct. 29, 2012 Lyside Sulfur - Kirgonia lyside Spiny Aster Guayacan or Soapbush √ Oct. 29, 2012 Orange Sulfur - Colias eurytheme Spiny Aster Legumes √ Sept. 26, 2007 Family Lycaenidae (Gossamer Winged) Cassius Blue - Leptotes cassius Spiny Aster Legumes √ Oct. 30, 2012 Ceraunus Blue - Hemiargus ceraunus Kidney wood Legumes- Acacia, Mesquite √ Oct. 30, 2012 Fatal Metalmark - Calephelis nemesis Spiny Aster Baccharis, Clematis √ Oct. 29, 2012 Gray Hairstreak - Strymon melinus Frostweed, Aster Many flowering plants √ Oct. 29, 2012 Great Purple Hairstreak - Atlides halesus Spiny Aster Mistletoe (in oak/mesquite) √ Oct. 29, 2012 Marine Blue - Leptotes marina Frogfruit Legumes- Acacia, Mesquite √ Oct. 30, 2012 Family Nymphalidae (Nymphalids) American Snout - Libytheana carinenta Spiny Aster Hackberries √ Oct. 29, 2012 Bordered Patch - Chlosyne lacinia Zexmenia Ragweed, Sunflower √ Oct. 29, 2012 Common Mestra - Mestra amymone Frostweed Noseburn (Tragia spp.) X Oct. 29, 2012 Empress Leila - Asterocampa leilia Spiny Aster Spiny Hackberry √ Oct. 29, 2012 Gulf Fritillary - Agraulis vanillae Spiny Aster Passion Vine X Oct. 29, 2012 Hackberry Emperor - Asterocampa celtis spiny Hackberry Hackberries √ Oct. 29, 2012 Painted Lady - Vanessa cardui Pink Smartweed Mallows & Thistles √ Oct. 29, 2012 Pearl Crescent - Phycoides tharos Spiny Aster Asters √ Oct. -

Butterflies in the Verde Valley
Butterflies in the Verde Valley 1 2 17 1. Mormon Fritillary, Speyeria mormonia 2. Empress Leilia, Asterocampa leilia 3. Fiery Skipper, Hylephila phyleus 4. Greenish-Blue Lycaenid, Plebejus saepiolus 16 4 18 Female on top, male below 3 5. Pipevine Swallowtail, Battus philenor 6. American Snout Butterfly, Libytheana carinenta 7. Cloudless Sulpher, Phoebis sennae 14 15 Female with patterned wing, male solid 5 8. Two-tailed Swallowtail, Papilio multicaudata 9. Variegated Fritillary, Euptoieta claudia 13 12 10. Hoary Comma, Polygonia gracilis 6 11. California Sister, Adelpha bredowii Eurema nicippe 8 12. Sleepy Orange Sulphur, 11 Two males 10 13. Alfalfa Sulfpur, Colias euretheme Two males, one female (pale) 14. Painted Lady, Vanessa cardui 15. Pine White, Neophasia menapia 9 7 16. Viceroy, Limenitus archippus 17. Queen, Danaus gilippus 18. Black Swallowtail, Papilio polyxenes Butterflies Butterflies are an amazing group of insects, and their delicate structure, flight, and colors, brighten our day. The butterflies in this display represent 6 related families of Lepidoptera (scaled wings) which we know as butterflies. All of these occur in northern Arizona. The best place to look for butterflies is often in moist creek beds and in areas where flowers are blooming. The mouth is composed of two long flexible straws that are connected. When they feed/drink they unroll the mouth parts (called a proboscis) and suck in nectar or other liquids. Although the shapes and colors differs, their basic structure is similar. All have four wings, six legs, an abdomen, thorax, head, and antenna. Butterflies have complete metamorphosis, with four separate stages: (1)egg, (2)larva, (3)chrysalis, and (4)adult. -

Global Journal of Science Frontier Research: C Biological Science Botany & Zology
Online ISSN : 2249-4626 Print ISSN : 0975-5896 DOI : 10.17406/GJSFR DiversityofButterflies RevisitingMelaninMetabolism InfluenceofHigh-FrequencyCurrents GeneticStructureofSitophilusZeamais VOLUME20ISSUE4VERSION1.0 Global Journal of Science Frontier Research: C Biological Science Botany & Zology Global Journal of Science Frontier Research: C Biological Science Botany & Zology Volume 20 Issue 4 (Ver. 1.0) Open Association of Research Society Global Journals Inc. © Global Journal of Science (A Delaware USA Incorporation with “Good Standing”; Reg. Number: 0423089) Frontier Research. 2020 . Sponsors:Open Association of Research Society Open Scientific Standards All rights reserved. This is a special issue published in version 1.0 Publisher’s Headquarters office of “Global Journal of Science Frontier Research.” By Global Journals Inc. Global Journals ® Headquarters All articles are open access articles distributed 945th Concord Streets, under “Global Journal of Science Frontier Research” Framingham Massachusetts Pin: 01701, Reading License, which permits restricted use. United States of America Entire contents are copyright by of “Global USA Toll Free: +001-888-839-7392 Journal of Science Frontier Research” unless USA Toll Free Fax: +001-888-839-7392 otherwise noted on specific articles. No part of this publication may be reproduced Offset Typesetting or transmitted in any form or by any means, electronic or mechanical, including G lobal Journals Incorporated photocopy, recording, or any information storage and retrieval system, without written 2nd, Lansdowne, Lansdowne Rd., Croydon-Surrey, permission. Pin: CR9 2ER, United Kingdom The opinions and statements made in this book are those of the authors concerned. Packaging & Continental Dispatching Ultraculture has not verified and neither confirms nor denies any of the foregoing and no warranty or fitness is implied. -

SYSTEMATICS of VAGRANTINI BUTTERFLIES (LEPIDOPTERA: Nymphalidae)
Treubia 2003 33 (1) 71-87 SYSTEMATICS OF VAGRANTINI BUTTERFLIES (LEPIDOPTERA: NYMPHAlIDAE). PART 1. CLADISTIC ANALYSIS Djunijanti Peggie . Division of Zoology, Research Center for Biology, Indonesian Institute of Sciences JI. Raya Jakarta Bogor Km. 46, Cibinong 16911, Indonesia Abstract Eiglit ge/lera of lndo-Australian butterjiies: Algia. Algiachroa, Cirrochroa, Cupha, Phalanta, Terinos, Vagrans, and Vindula are presented here. These genera together with two Afrotropical genera: Lachnoptera and Smerina, and a Central American genlls Euptoieta were previollsly placed as subiribe uncertain. One-hundred adult morphological characters were scored for fifty-four taxa, and were analyzed simultaneousuj (Nixon and Carpenter, 1993). The cladistic analysis showed that all species were properly assigned to monophyletic genera, and the arrangement of the outgroup taxa is in concordance with the classification previously suggested. The eight lndo-Australian and two Afrotropical genera belong to the tribe Vagrantini within the subfamily Heliconiinae. Key words: Heliconiines, Vagrantini, Indo-Australian, butterflies. Introduction The subfamily Heliconiinae is recognized by most authorities but the included taxa may differ. Ackery (in Vane-Wright and Ackery, 1984) suggested that the heliconiines may prove to represent a highly specialized subgroup of the Argynnini sensu lato. Heliconiinae sensu Harvey (in Nijhout, 1991) also include Acraeinae and Argynninae of Ackery (1988).Parsons (1999)included argynnines within Heliconiinae but retained Acraeinae as a distinct subfamily. Harvey (in N ijhou t, 1991) recognized three tribes of Heliconiinae: Pardopsini, Acraeini, and Heliconiini. The Heliconiini include the Neotropical Heliconiina (Brower, 2000), some genera which were placed as "subtribe uncertain", Argynnina, Boloriina and three other genera (the Neotropical genusYramea, the Oriental Kuekenthaliella, and Prokuekenthaliella) with uncertain relationships. -

Great Basin Silverspot Butterfly (Speyeria Nokomis Nokomis [W.H
Great Basin Silverspot Butterfly (Speyeria nokomis nokomis [W.H. Edwards]): A Technical Conservation Assessment Prepared for the USDA Forest Service, Rocky Mountain Region, Species Conservation Project April 25, 2007 Gerald Selby Ecological and GIS Services 1410 W. Euclid Ave. Indianola, IA 50125 Peer Review Administered by Society for Conservation Biology Selby, G. (2007, April 25). Great Basin Silverspot Butterfly (Speyeria nokomis nokomis [W.H. Edwards]): a technical conservation assessment. [Online]. USDA Forest Service, Rocky Mountain Region. Available: http://www.fs.fed.us/r2/projects/scp/assessments/greatbasinsilverspotbutterfly.pdf [date of access]. ACKNOWLEDGMENTS This assessment was prepared for the Rocky Mountain Region of the USDA Forest Service under contract number 53-82X9-4-0074. I would like to express my appreciation to the USDA Forest Service staff with whom I worked on the project: Richard Vacirca reviewed earlier drafts, Gary Patton provided valuable general oversight for the project and suggested improvements for the final draft, John Sidle reviewed the final draft, Nancy McDonald provided final editorial review of the pre-publication manuscript, and Kimberly Nguyen prepared the manuscript for Web publication. The entire staff, including Richard Braun, the contracting officer, provided the flexibility and contract extensions needed to bring this assessment to final publication. Two anonymous reviewers also provided valuable feedback. Their careful review and editorial input helped to improve the quality of the final product greatly. I would also like to express my appreciation to Natural Heritage Program personnel and other experts (professional and amateur) that took time from their busy schedules to provide information and data needed to complete this assessment.