Influence of Inhalation/Exhalation Ratio and of Respiratory
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
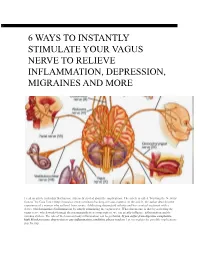
6 Ways to Instantly Stimulate Your Vagus Nerve to Relieve Inflammation, Depression, Migraines and More
O 6 WAYS TO INSTANTLY STIMULATE YOUR VAGUS NERVE TO RELIEVE INFLAMMATION, DEPRESSION, MIGRAINES AND MORE I read an article yesterday that has me extremely excited about the implications. The article is called “Hacking the Nervous System” by Gaia Vince (http://mosaicscience.com/story/hacking-nervous-system). In the article, the author describes the experience of a woman who suffered from severe, debilitating rheumatoid arthritis and her eventual treatment with a device which minimized inflammation by simply stimulating the vagus nerve. What this means, is that by activating the vagus nerve which works through the parasympathetic nervous system, we can greatly influence inflammation and the immune system. The role of the brain on body inflammation can be profound. If you suffer from digestive complaints, high blood pressure, depression or any inflammatory condition, please read on. Let me explain the possible implications step by step. What is the vagus nerve? First of all, the vagus nerve is the longest nerve in the body which originates in the brain as cranial nerve ten, travels down the from go the neck and then passes around the digestive system, liver, spleen, pancreas, heart and lungs. This nerve is a major player in the parasympathetic nervous system, which is the ‘rest and digest’ part (opposite to the sympathetic nervous system which is ‘fight of flight’). Vagal tone The tone of the vagus nerve is key to activating the parasympathetic nervous system. Vagal tone is measured by tracking your heart-rate alongside your breathing rate. Your heart-rate speeds up a little when your breathe in, and slows down a little when you breathe out. -

Peak Flow Measure: an Index of Respiratory Function?
International Journal of Health Sciences and Research www.ijhsr.org ISSN: 2249-9571 Original Research Article Peak Flow Measure: An Index of Respiratory Function? D. Devadiga, Aiswarya Liz Varghese, J. Bhat, P. Baliga, J. Pahwa Department of Audiology and Speech Language Pathology, Kasturba Medical College (A Unit of Manipal University), Mangalore -575001 Corresponding Author: Aiswarya Liz Varghese Received: 06/12/2014 Revised: 26/12/2014 Accepted: 05/01/2015 ABSTRACT Aerodynamic analysis is interpreted as a reflection of the valving activity of the larynx. It involves measuring changes in air volume, flow and pressure which indicate respiratory function. These measures help in determining the important aspects of lung function. Peak expiratory flow rate is a widely used respiratory measure and is an effective measure of effort dependent airflow. Aim: The aim of the current study was to study the peak flow as an aerodynamic measure in healthy normal individuals Method: The study group was divided into two groups with n= 60(30 males and 30 females) in the age range of 18-22 years. The peak flow was measured using Aerophone II (Voice Function Analyser). The anthropometric measurements such as height, weight and Body Mass Index was calculated for all the participants. Results: The peak airflow was higher in females as compared to that of males. It was also observed that the peak air flow rate was correlating well with height and weight in males. Conclusions: Speech language pathologist should consider peak expiratory airflow, a short sharp exhalation rate as a part of routine aerodynamic evaluation which is easier as compared to the otherwise commonly used measure, the vital capacity. -

Spirometry Basics
SPIROMETRY BASICS ROSEMARY STINSON MSN, CRNP THE CHILDREN’S HOSPITAL OF PHILADELPHIA DIVISION OF ALLERGY AND IMMUNOLOGY PORTABLE COMPUTERIZED SPIROMETRY WITH BUILT IN INCENTIVES WHAT IS SPIROMETRY? Use to obtain objective measures of lung function Physiological test that measures how an individual inhales or exhales volume of air Primary signal measured–volume or flow Essentially measures airflow into and out of the lungs Invaluable screening tool for respiratory health compared to BP screening CV health Gold standard for diagnosing and measuring airway obstruction. ATS, 2005 SPIROMETRY AND ASTHMA At initial assessment After treatment initiated and symptoms and PEF have stabilized During periods of progressive or prolonged asthma control At least every 1-2 years: more frequently depending on response to therapy WHY NECESSARY? o To evaluate symptoms, signs or abnormal laboratory tests o To measure the effect of disease on pulmonary function o To screen individuals at risk of having pulmonary disease o To assess pre-operative risk o To assess prognosis o To assess health status before beginning strenuous physical activity programs ATS, 2005 SPIROMETRY VERSUS PEAK FLOW Recommended over peak flow meter measurements in clinician’s office. Variability in predicted PEF reference values. Many different brands PEF meters. Peak Flow is NOT a diagnostic tool. Helpful for monitoring control. EPR 3, 2007 WHY MEASURE? o Some patients are “poor perceivers.” o Perception of obstruction variable and spirometry reveals obstruction more severe. o Family members “underestimate” severity of symptoms. o Objective assessment of degree of airflow obstruction. o Pulmonary function measures don’t always correlate with symptoms. o Comprehensive assessment of asthma. -

Characterisation of Breathing and Associated Central Autonomic
Arch Dis Child 2001;85:29–37 29 Characterisation of breathing and associated Arch Dis Child: first published as 10.1136/adc.85.1.29 on 1 July 2001. Downloaded from central autonomic dysfunction in the Rett disorder P O O Julu, A M Kerr, F Apartopoulos, S Al-Rawas, I Witt Engerström, L Engerström, G A Jamal, S Hansen Abstract is associated with non-epileptic vacant spells.11 Aim—To investigate breathing rhythm Low resting cardiac vagal tone and weak vagal and brain stem autonomic control in response to hyperventilation and breath hold- patients with Rett disorder. ing suggest inadequate parasympathetic con- Setting—Two university teaching hospi- trol.12 tals in the United Kingdom and the Rett Our aim in this study was to characterise the Centre, Sweden. abnormalities of respiratory rhythm and inves- Patients—56 female patients with Rett tigate the central autonomic competence in disorder, aged 2–35 years; 11 controls aged Rett disorder. 5–28 years. Design—One hour recordings of breath- ing movement, blood pressure, ECG R-R Methods interval, heart rate, transcutaneous blood SUBJECTS Fifty six subjects were referred for diagnostic gases, cardiac vagal tone, and cardiac assessment. Control values came from 11 sensitivity to baroreflex measured on-line female volunteers and from previous with synchronous EEG and video. Breath- studies.12–14 Parents received written explana- ing rhythms were analysed in 47 cases. tions of procedures and results and provided Results—Respiratory rhythm was normal consent. The ethics committee at South during sleep and abnormal in the waking Glasgow University Hospitals NHS Trust state. -

NIOSH), Centers for Disease Control and Prevention (CDC)
Technical Report Filtering Facepiece Respirators with an Exhalation Valve: Measurements of Filtration Efficiency to Evaluate Their Potential for Source Control Centers for Disease Control and Prevention National Institute for Occupational Safety and Health Technical Report Filtering Facepiece Respirators with an Exhalation Valve: Measurements of Filtration Efficiency to Evaluate Their Potential for Source Control DEPARTMENT OF HEALTH AND HUMAN SERVICES Centers for Disease Control and Prevention National Institute for Occupational Safety and Health National Personal Protective Technology Laboratory This document is in the public domain and may be freely copied or reprinted. Disclaimer Mention of any company or product does not constitute endorsement by the National Institute for Occupational Safety and Health (NIOSH), Centers for Disease Control and Prevention (CDC). In addition, citations to websites external to NIOSH do not constitute NIOSH endorsement of the sponsoring organizations or their programs or products. Furthermore, NIOSH is not responsible for the content of these websites. All web addresses referenced in this document were accessible as of the publication date. Get More Information Find NIOSH products and get answers to workplace safety and health questions: 1-800-CDC-INFO (1-800-232-4636) | TTY: 1-888-232-6348 CDC/NIOSH INFO: cdc.gov/info | cdc.gov/niosh Monthly NIOSH eNews: cdc.gov/niosh/eNews Suggested Citation NIOSH [2020]. Filtering facepiece respirators with an exhalation valve: measurements of filtration efficiency to evaluate their potential for source control. By Portnoff L, Schall J, Brannen J, Suhon N, Strickland K, Meyers J. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, DHHS (NIOSH) Publication No. -

Physical and Geometric Constraints Shape the Labyrinth-Like Nasal Cavity
Physical and geometric constraints shape the labyrinth-like nasal cavity David Zwickera,b,1, Rodolfo Ostilla-Monico´ a,b, Daniel E. Liebermanc, and Michael P. Brennera,b aJohn A. Paulson School of Engineering and Applied Sciences, Harvard University, Cambridge, MA 02138; bKavli Institute for Bionano Science and Technology, Harvard University, Cambridge, MA 02138; and cDepartment of Human Evolutionary Biology, Harvard University, Cambridge, MA 02138 Edited by Leslie Greengard, New York University, New York, NY, and approved January 26, 2018 (received for review August 29, 2017) The nasal cavity is a vital component of the respiratory system take into account geometric constraints imposed by the shape that heats and humidifies inhaled air in all vertebrates. Despite of the head that determine the length of the nasal cavity, its this common function, the shapes of nasal cavities vary widely cross-sectional area, and, generally, the shape of the space that it across animals. To understand this variability, we here connect occupies. To tackle this complex problem, we first show that, nasal geometry to its function by theoretically studying the air- without geometric constraints, optimal shapes have slender flow and the associated scalar exchange that describes heating cross-sections. We then demonstrate that these shapes can be and humidification. We find that optimal geometries, which have compacted into the typical labyrinth-like shapes without much minimal resistance for a given exchange efficiency, have a con- loss in performance. stant gap width between their side walls, while their overall shape can adhere to the geometric constraints imposed by the Results head. Our theory explains the geometric variations of natural The Flow in the Nasal Cavity Is Laminar. -

Standardisation of Spirometry
Eur Respir J 2005; 26: 319–338 DOI: 10.1183/09031936.05.00034805 CopyrightßERS Journals Ltd 2005 SERIES ‘‘ATS/ERS TASK FORCE: STANDARDISATION OF LUNG FUNCTION TESTING’’ Edited by V. Brusasco, R. Crapo and G. Viegi Number 2 in this Series Standardisation of spirometry M.R. Miller, J. Hankinson, V. Brusasco, F. Burgos, R. Casaburi, A. Coates, R. Crapo, P. Enright, C.P.M. van der Grinten, P. Gustafsson, R. Jensen, D.C. Johnson, N. MacIntyre, R. McKay, D. Navajas, O.F. Pedersen, R. Pellegrino, G. Viegi and J. Wanger CONTENTS AFFILIATIONS Background ............................................................... 320 For affiliations, please see Acknowledgements section FEV1 and FVC manoeuvre .................................................... 321 Definitions . 321 CORRESPONDENCE Equipment . 321 V. Brusasco Requirements . 321 Internal Medicine University of Genoa Display . 321 V.le Benedetto XV, 6 Validation . 322 I-16132 Genova Quality control . 322 Italy Quality control for volume-measuring devices . 322 Fax: 39 103537690 E-mail: [email protected] Quality control for flow-measuring devices . 323 Test procedure . 323 Received: Within-manoeuvre evaluation . 324 March 23 2005 Start of test criteria. 324 Accepted after revision: April 05 2005 End of test criteria . 324 Additional criteria . 324 Summary of acceptable blow criteria . 325 Between-manoeuvre evaluation . 325 Manoeuvre repeatability . 325 Maximum number of manoeuvres . 326 Test result selection . 326 Other derived indices . 326 FEVt .................................................................. 326 Standardisation of FEV1 for expired volume, FEV1/FVC and FEV1/VC.................... 326 FEF25–75% .............................................................. 326 PEF.................................................................. 326 Maximal expiratory flow–volume loops . 326 Definitions. 326 Equipment . 327 Test procedure . 327 Within- and between-manoeuvre evaluation . 327 Flow–volume loop examples. 327 Reversibility testing . 327 Method . -

Vagal Tone Regulates Cardiac Shunts During Activity and at Low Temperatures in the South American Rattlesnake, Crotalus Durissus
J Comp Physiol B (2016) 186:1059–1066 DOI 10.1007/s00360-016-1008-y ORIGINAL PAPER Vagal tone regulates cardiac shunts during activity and at low temperatures in the South American rattlesnake, Crotalus durissus Renato Filogonio1,2 · Tobias Wang2 · Edwin W. Taylor1,3 · Augusto S. Abe1 · Cléo A. C. Leite4 Received: 2 February 2016 / Revised: 18 May 2016 / Accepted: 3 June 2016 / Published online: 13 June 2016 © Springer-Verlag Berlin Heidelberg 2016 Abstract The undivided ventricle of non-crocodilian rep- pulmonary and systemic blood flow in both groups, but tiles allows for intracardiac admixture of oxygen-poor and net cardiac shunt was reversed in the vagotomized group oxygen-rich blood returning via the atria from the sys- at lower temperatures. We conclude that vagal control of temic circuit and the lungs. The distribution of blood flow pulmonary conductance is an active mechanism regulating between the systemic and pulmonary circuits may vary, cardiac shunts in C. durissus. based on differences between systemic and pulmonary vas- cular conductances. The South American rattlesnake, Cro- Keywords Reptiles · Snakes · Cardiac shunt · Vagus talus durissus, has a single pulmonary artery, innervated nerve · Arterial pressure · Blood flow · Vascular regulation by the left vagus. Activity in this nerve controls pulmonary conductance so that left vagotomy abolishes this control. Experimental left vagotomy to abolish cardiac shunting Introduction had no effect on long-term survival and failed to identify a functional role in determining metabolic rate, growth or The undivided ventricle of the non-crocodilian reptile resistance to food deprivation. Accordingly, the present heart enables variable proportions of cardiac output to investigation sought to evaluate the extent to which car- bypass the systemic or pulmonary circulations, resulting diac shunt patterns are actively controlled during changes in either left-to-right (L–R) or right-to-left (R–L) cardiac in body temperature and activity levels. -

A Review of the Breathing Mechanism for Singing
A Review of the Breathing Mechanism for Singing: Part I: Anatomy Dr. Sean McCarther Breathing is important for singing. In fact, many argue that a properly coordinated breathing mechanism is one of, if not the most important components of a vocal technique. As my former teacher, Dr. Robert Harrison, is fond of saying, “No air, no sound.” It is our responsibility as teachers of singing to help students learn to coordinate the muscles and organs of the breathing mechanism in such a way as to produce the most efficient and vibrant sound. Part I of this series will focus on the anatomy of the breathing mechanism. It will review the major organs, muscles, and bones and discuss how they interact with each other for both passive and active breathing. Part II will examine the singer’s breath in greater detail and discuss various ways the system can be used for singing, including a description of appoggio. The Lungs and Lower Airway The lungs are made of a porous, spongy material that is somewhat elastic in nature (its elasticity is an important part of exhalation, but more on that later). The lungs attach to the ribs via the pleural sacs, two thin pieces of membrane that cause the lungs to adhere to the ribs. Because of this connection, any change in the volume of the rib cage causes a similar change in the volume of the lungs. As the volume of the lungs increase, a vacuum is created, causing air to rush in and fill the lungs. This is called inhalation. -

Breathing of Humans and Its Simulation
Breathing of Humans and its Simulation Mina Nishi LSTM-Erlangen Institute of Fluid Mechanics Friedlich-Alexander-University Erlangen-Nuremberg Cauerstr.4, D-91058 Erlangen June 14, 2004 Abstract In this thesis, a breathing flow rate monitoring system and a mechanical breathing simulator are developed, constructed and tested for the experimental investiga- tion of human breathing. Human breathing is a combination of three lung functions, ventilation, diffusion and circulation. In the present thesis, ventilation functioning is focused for the measurment and simulation. One of the most interesting features of ventilation functioning is its time varying volume flow rate. To measure this, the breathing mask which covers nose and mouth are used with cooperating with the thermal sensor. The sensor is called Time of Flight sensor, which is environmental con- dition, like temperature or humidity, independent and direction sensitive. The sensor gives two different information, one is the direction signal and the other signal is proportional to the mass flow rate. With this system, the ventilation volume flow rate can be precisely measured so that the exact human ventilation simulation will be realized. The sampled raw data of human ventilation will be analyzed to obtain the typical ventilation curve which is used for diagnosis of lung functioning defection. The second important part of this thesis is to simulate human ventilation with certain equipment which can reproduce any kind of ventilation curve. The simulation system is constructed with the mass flow contoroller which is applied for the exhalation simulation and for the inhalation simulation, volume flow con- troller, a proportional valve which is operated with vacuum pump and chamber. -

Fetal Heart Rate Tracing Study
FHR Monitoring: Maternal Fetal Physiology M. Sean Esplin, MD and Alexandra Eller, MD Maternal Fetal Medicine Intermountain Healthcare University of Utah Health Sciences Center Disclosures • I have no financial relationships to disclose • I want to acknowledge ACOG District II for open sharing of their teaching modules http://www.acog.org/About-ACOG/ACOG-Districts/District- II/Quick-Guide-and-Teaching-Modules Goals • Review the basic physiology and adaptive responses that regulate the FHR • Review oxygen delivery to the fetus, potential disruptions, and route to injury • Correlate physiologic fetal adaptations to stress with FHR deceleration patterns Goal of Intrapartum Monitoring Assess the adequacy of intrapartum fetal oxygenation • Reduce perinatal morbidity and mortality • Perinatal death • Intrapartum and neonatal • Birth asphyxia • Long-term neurological impairment The Basic Assumption Fetal adaptive responses to progressive hypoxemia and acidosis are detectable FHR monitoring = fetal brain oxygenation monitoring Basic Heart Rate Physiology • Cardiorespiratory Center (medulla oblongata) • Determines FHR baseline, variability, pattern • Coordinates input from intrinsic influences • Parasympathetic nervous system • Sympathetic nervous system • Baroreceptors (aortic arch, carotid) • Chemoreceptors (central and peripheral) • Endocrine system (hormones) • Sleep-wake cycle • Breathing/Pain/Sound/Temperature http://images.slideplayer.com/25/7741495/slides/slide_24.jpg Parasympathetic Influence • Vagus (CN X) nerve originates in CRC • Innervates -

TIPPS for Calming Down Sooner
TIPPS for Calming Down Sooner DBT skills offer us the following “TIPPS” to help our bodies help us calm down. T – Temperature – cold exposure, especially head and face I – Intense Exercise – short high intensity activity P – Paced Breathing - deep belly breath, slow full exhale P – Progressive Muscle Relaxation- Squeeze and release 1 by 1 S – Sing, Hum, or Chant (or gargle) MORE ABOUT WHY THIS MIGHT HELP From Optimal Living Dynamics (https://www.optimallivingdynamics.com/blog/how-to- stimulate-your-vagus-nerve-for-better-mental-health-brain-vns-ways-treatment-activate-natural- foods-depression-anxiety-stress-heart-rate-variability-yoga-massage-vagal-tone-dysfunction) It seems there is more information on this topic all the time. If interested check it out. Here are some short thoughts. The vagus nerve is the longest cranial nerve in our body. It connects our brain to many important organs throughout the body, including the gut (intestines, stomach), heart and lungs. The vagus nerve is also a key part of our parasympathetic “rest and digest” nervous system. It influences our breathing, digestive function and heart rate, all of which can have a huge impact on our mood and mental health. Special attention should be paid to the "tone" of our vagus nerve. Increasing vagal tone activates the parasympathetic nervous system, and having higher vagal tone means that our body can relax faster after stress. TRY SOME OR ALL OF THESE IN-HOME STEPS TO INCREASE VAGUS NERVE STIMULATION NATURALLY: 1. Cold Exposure Try finishing your next shower with at least 30 seconds of cold water and see how you feel.