Veterinary Endoscopy – Small Animals –
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Product Catalog Stainless Steel Vaginal Specula
PRODUCT CATALOG STAINLESS STEEL VAGINAL SPECULA Graves Speculum Product No. Description LTL-GS300 Graves Speculum, Small 3” x .75” LTL-GS400 Graves Speculum, Medium 4” x 1.5” LTL-GS450 Graves Speculum, Large 4.50” x 1.5” LTL-GS700 Graves Speculum, XL 7” x 1.5” Pederson Speculum Product No. Description LTL-PS305 Pederson Speculum, Virginal 3” x .5” LTL-PS300 Pederson Speculum, Small 3” x 1” LTL-PS400 Pederson Speculum, Medium 4” x 1” LTL-PS450 Pederson Speculum, Large 4.5” x 1” LTL-PS455 Pederson Speculum, Extra Narrow 4.5” x .5” LTL-PS700 Pederson Speculum, XL 7” x 1” Open Sided Speculum Product No. Description LTL-WGR400 Weisman-Graves Speculum, Medium, Right Open 4” x 1.5” LTL-WGR450 Weisman-Graves Speculum, Large, Right Open 4.5” x 1.5” LTL-WGL400 Weisman-Graves Speculum, Medium, Left Open 4” x 1.5” LTL-WGL450 Weisman-Graves Speculum, Large, Left Open 4.5” x 1.5” LTL-WPR400 Weisman-Pederson Speculum, Medium, Right Open 4” x 1” LTL-WPR450 Weisman-Pederson Speculum, Large, Right Open 4.5” x 1” LTL-WPL400 Weisman-Pederson Speculum, Medium, Left Open 4” x 1” LTL-WPL450 Weisman-Prderspm Speculum, Large, Left Open 4.5” x 1” *We also offer wide view (4cm) and full view (7cm) openings. 1 | TOLL FREE 1 [800] 910-8303 FAX 1 [805] 579-9415 WWW.LTLMEDICAL.NET BIOPSY PUNCHES Standard Style Rotating Style Tischler [Morgan] 7mm x 3mm Baby Tischler 4mm x 2mm Tischler Kevorkian 9.5mm x 3mm Product No. Description Product No. Description Product No. -
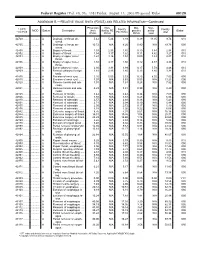
RELATIVE VALUE UNITS (RVUS) and RELATED INFORMATION—Continued
Federal Register / Vol. 68, No. 158 / Friday, August 15, 2003 / Proposed Rules 49129 ADDENDUM B.—RELATIVE VALUE UNITS (RVUS) AND RELATED INFORMATION—Continued Physician Non- Mal- Non- 1 CPT/ Facility Facility 2 MOD Status Description work facility PE practice acility Global HCPCS RVUs RVUs PE RVUs RVUs total total 42720 ....... ........... A Drainage of throat ab- 5.42 5.24 3.93 0.39 11.05 9.74 010 scess. 42725 ....... ........... A Drainage of throat ab- 10.72 N/A 8.26 0.80 N/A 19.78 090 scess. 42800 ....... ........... A Biopsy of throat ................ 1.39 2.35 1.45 0.10 3.84 2.94 010 42802 ....... ........... A Biopsy of throat ................ 1.54 3.17 1.62 0.11 4.82 3.27 010 42804 ....... ........... A Biopsy of upper nose/ 1.24 3.16 1.54 0.09 4.49 2.87 010 throat. 42806 ....... ........... A Biopsy of upper nose/ 1.58 3.17 1.66 0.12 4.87 3.36 010 throat. 42808 ....... ........... A Excise pharynx lesion ...... 2.30 3.31 1.99 0.17 5.78 4.46 010 42809 ....... ........... A Remove pharynx foreign 1.81 2.46 1.40 0.13 4.40 3.34 010 body. 42810 ....... ........... A Excision of neck cyst ........ 3.25 5.05 3.53 0.25 8.55 7.03 090 42815 ....... ........... A Excision of neck cyst ........ 7.07 N/A 5.63 0.53 N/A 13.23 090 42820 ....... ........... A Remove tonsils and ade- 3.91 N/A 3.63 0.28 N/A 7.82 090 noids. -

General Reprocessing Instructions for KARL STORZ Products (USA)
General Reprocessing Instructions for KARL STORZ Products (USA) Important information for users of KARL STORZ instruments Please read this entire instructions-for-use carefully before using the KARL STORZ instruments. Failure to follow the instructions, cautions and warnings presented in this manual may result in serious consequences to the patient. Procedures for proper handling and care of KARL STORZ instruments are described in this instructions-for-use. KARL STORZ endoscopes and accessories are delicate surgical instruments and should be handled with care. Improper use during surgical procedures will result in damage, breakage or patient injury. KARL STORZ Endoscopy-America, Inc. assumes no liability if the instruments are misused, mishandled or otherwise abused. Proper handling and care, as described in this instructions-for-use, will prolong the life of these instruments Contents Important Information 1 Why is Water Quality Important? Water quality requirements 2 Table 1 – General Sterilization and High Level Disinfection Matrix 3 Table 2 – Camera Heads Sterilization and High Level Disinfection Matrix 4 Pre-Cleaning Preparation 5 Cleaning Instructions for Instruments 5 - 7 Table 3 – Cleaning brushes 6 Sterilization Instructions (general introduction) 7 Ethylene oxide (EtO) Sterilization 8 - 9 Table 4 – EtO Cycle Parameters 9 Steam 10 - 11 STERRAD Sterilization Systems 12 - 15 Table 5 – STERRAD Sterilization Systems Lumen Claims 15 Table 6 – STERRAD Tray Compatibility and Maximum Load Matrix 16 STERIS Amsco V-PRO Sterilization Systems 17 High Level Disinfection 18 - 20 Note: This document is updated frequently. Please check for latest update. KARL STORZ 2151 E. Grand Avenue Phone 424 218 8100 Endoscopy-America, Inc. El Segundo, CA 90245-5071 Toll Free 800 421 0837 PI-000035-20.1 2-03-11 General Reprocessing Instructions for KARL STORZ Products (USA) WHY IS WATER QUALITY IMPORTANT? The quality of water used for instrument processing has a considerable influence on the proper function and longevity of an instrument. -

Endo Product Guide 2018
Visualization Plastic and Reconstructive Surgery Arthroscopy Urology Gynecology Laparoscopy Services U.S. Product Guide 2018 Visualization Plastic and Arthroscopy 12 Camera systems and Reconstructive 40 Arthroscopes minimally invasive surgery Surgery 44 Arthroscope hardware 19 Open visualization 34 SPY-PHI Fluorescence 50 Powered instruments 20 Video enhancement Imaging System 53 Cutters & burs 21 Light source 35 SPY Elite Fluorescence Imaging System 59 Arthroscopy pumps 24 Data management 36 DermACELL Acellular 61 Single portal arthroscopy 27 Printers Dermal Matrix (ADM) 63 Hip arthroscopy 28 Flat panel surgical display 69 Small-joint arthroscopy 30 Wireless technology 71 Manual instruments 31 Video carts 74 Disposable cannulas 75 Micro cart Urology Laparoscopy Services 78 Rigid cystoscopes 96 Laparoscopes 116 OnSite Services 80 Resection 99 Manual instruments 117 ProCare Services 82 Laser cystoscope 106 Electrosurgical instruments 118 Replacement parts 83 Urethrotome 107 Suction Irrigation 120 Repair process 84 Ureteroscopes 110 Insuffators 121 Loaner and replacement policy 85 Urology accessories 112 ICG and infrared technology 113 Laparoscopic Gynecology training system Index 88 Rigid hysteroscopes 113 Pneumatic scope holder 122 Index 90 Resection 93 Gynecologic laparoscopy instruments 93 Fluid management We are This mission is at the heart of what we do and believe. We collaborate with our customers to develop innovative products and services that ultimately improve the lives of patients. We understand that in today’s challenging healthcare environment, our customers need solutions that Stryker improve quality and effciency in the operating room and, ultimately, the patient experience. These challenges are creating opportunities for us to Together with our customers, make healthcare better, for our customers and the we are driven to make healthcare better. -

Answer Key Chapter 1
Instructor's Guide AC210610: Basic CPT/HCPCS Exercises Page 1 of 101 Answer Key Chapter 1 Introduction to Clinical Coding 1.1: Self-Assessment Exercise 1. The patient is seen as an outpatient for a bilateral mammogram. CPT Code: 77055-50 Note that the description for code 77055 is for a unilateral (one side) mammogram. 77056 is the correct code for a bilateral mammogram. Use of modifier -50 for bilateral is not appropriate when CPT code descriptions differentiate between unilateral and bilateral. 2. Physician performs a closed manipulation of a medial malleolus fracture—left ankle. CPT Code: 27766-LT The code represents an open treatment of the fracture, but the physician performed a closed manipulation. Correct code: 27762-LT 3. Surgeon performs a cystourethroscopy with dilation of a urethral stricture. CPT Code: 52341 The documentation states that it was a urethral stricture, but the CPT code identifies treatment of ureteral stricture. Correct code: 52281 4. The operative report states that the physician performed Strabismus surgery, requiring resection of the medial rectus muscle. CPT Code: 67314 The CPT code selection is for resection of one vertical muscle, but the medial rectus muscle is horizontal. Correct code: 67311 5. The chiropractor documents that he performed osteopathic manipulation on the neck and back (lumbar/thoracic). CPT Code: 98925 Note in the paragraph before code 98925, the body regions are identified. The neck would be the cervical region; the thoracic and lumbar regions are identified separately. Therefore, three body regions are identified. Correct code: 98926 Instructor's Guide AC210610: Basic CPT/HCPCS Exercises Page 2 of 101 6. -

THE BONFILS INTUBATION ENDOSCOPE in Clinical and Emergency Medicine
® THE BONFILS INTUBATION ENDOSCOPE in Clinical and Emergency Medicine Tim PIEPHO Rüdiger NOPPENS ® THE BONFILS INTUBATION ENDOSCOPE in Clinical and Emergency Medicine Tim PIEPHO Rüdiger NOPPENS Department of Anesthesiology University Medical Center of the Johannes Gutenberg University Mainz, Germany With contributions from: Andreas THIERBACH Rita METZ Pedro BARGON 4 The BONFILS Intubation Endoscope in Clinical and Emergency Medicine Important notes: The BONFILS Intubation Endoscope Medical knowledge is ever changing. As new research in Clinical and Emergency Medicine and clinical experience broaden our knowledge, Tim Piepho and Rüdiger Noppens changes in treat ment and therapy may be required. Department of Anesthesiology, The authors and editors of the material herein have University Medical Center of the Johannes Gutenberg University Mainz, Germany consulted sources believed to be reliable in their efforts to provide information that is complete and in accord with the standards accept ed at the time of publication. However, in view of the possibili ty of human error by Correspondence address: the authors, editors, or publisher, or changes in medical Tim Piepho knowledge, neither the authors, editors, publisher, nor Klinik für Anästhesiologie any other party who has been involved in the preparation Universitätsmedizin der Johannes Gutenberg-Universität Mainz of this booklet, warrants that the information contained Langenbeckstr. 1 herein is in every respect accurate or complete, and they Telephone: +49 (0)6131/171 are not responsible for any errors or omissions or for the results obtained from use of such information. The E-mail: [email protected] information contained within this booklet is intended for use by doctors and other health care professionals. -

Olympus Uptime Guarantee
Olympus Uptime Guarantee At Olympus, we strive to provide our customers with products and services that help optimize equipment use – and our repair agreements are no exception. The Olympus Uptime Guarantee (OUG) is a no-cost enhancement to our comprehensive Full Service Agreement. It is designed to curb delays caused by out-of-service equipment, enabling you to maximize procedural uptime. OUG delivers unprecedented benefits to eligible* Full Service Agreement customers, including: Next-Day Replacement Guaranteed Service Expense Control OUG provides no hassle, If we are unable to provide Full Service Agreements next-day replacement for next-day replacement, already provide accidental surgical products in need Olympus will cover half of damage coverage and fixed of repair through our refurbishment-level charges monthly repair expenditures. Advanced Replace® upon inspection of the With OUG, your Full Service Program – guaranteed. out-of-service product. Agreement will reflect the specific repair required for your out-of-service product, rather than a pre-fixed charge. *Eligibility Requirements: Product must be listed on current Olympus Uptime Guarantee product list, and Product must be covered on a Full Service Agreement with a start date of 01/01/15 or later. Olympus Uptime Guarantee Follow Four Steps to Receive the Olympus Uptime Guarantee: 1. Call 1-800-848-9024 by 8PM EST, Monday – Friday, to request an Advanced Replacement. 2. Provide contact information, model, serial number, and other requested information to a Customer Solutions Representative. OUG eligibility will be confirmed. 3. Receive Advanced Replacement shipping confirmation and an RMA for your out-of-service product. 4. Upon receipt of Advanced Replacement, immediately send out-of-service product to Olympus. -

Optical Devices in Tracheal Intubation—State of the Art in 2020
diagnostics Review Optical Devices in Tracheal Intubation—State of the Art in 2020 Jan Matek 1,2, Frantisek Kolek 3, Olga Klementova 4, Pavel Michalek 5,6 and Tomas Vymazal 3,* 1 1st Department of Surgery—Department of Abdominal, Thoracic Surgery and Traumatology, First Faculty of Medicine, Charles University in Prague and General University Hospital in Prague, 12800 Prague, Czech Republic; [email protected] 2 Medical Faculty, Masaryk University, 62500 Brno, Czech Republic 3 Department of Anesthesiology and Intensive Medicine, University Hospital Motol, V Úvalu 84, 15000 Praha, Czech Republic; [email protected] 4 Department of Anesthesiology and Intensive Medicine, University Hospital Olomouc, I.P. Pavlova 185, Nová Ulice, 77900 Olomouc, Czech Republic; [email protected] 5 Department of Anesthesiology and Intensive Medicine, First Faculty of Medicine, Charles University in Prague and General University Hospital, U Nemocnice 499/2, 12808 Praha, Czech Republic; [email protected] 6 Department of Anaesthesia, Antrim Area Hospital, Antrim BT41 2RL, UK * Correspondence: [email protected]; Tel.: +420-606-413-489 Abstract: The review article is focused on developments in optical devices, other than laryngoscopes, in airway management and tracheal intubation. It brings information on advantages and limita- tions in their use, compares different devices, and summarizes benefits in various clinical settings. Supraglottic airway devices may be used as a conduit for fiberscope-guided tracheal intubation mainly as a rescue plan in the scenario of difficult or failed laryngoscopy. Some of these devices offer the possibility of direct endotracheal tube placement. Hybrid devices combine the features of two different intubating tools. -

Diagnostic and Therapeutic Push Type Enteroscopy in Clinical Use Gut: First Published As 10.1136/Gut.37.3.346 on 1 September 1995
346 Gut 1995; 37: 346-352 Diagnostic and therapeutic push type enteroscopy in clinical use Gut: first published as 10.1136/gut.37.3.346 on 1 September 1995. Downloaded from G R Davies, M J Benson, D J Gertner, R M N Van Someren, D S Rampton, C P Swain Abstract laser or bipolar diathermy. In conclusion, This study describes small bowel push push enteroscopy is a practical and enteroscopy in routine clinical practice, valuable clinical service, which should using a purpose designed instrument probably become available on a sub- (Olympus SIF-10). Fifty six patients had a regional basis. total of60 procedures over a two and a half (Gut 1995; 37: 346-352) year period. The median (range) depth of Keywords: enteroscopy, small intestine, small intestine intubated was 45 (15-90) gastrointestinal bleeding, enteral nutrition. cm. Procedure time varied from 10-45 minutes. Most enteroscopies were performed during routine gastroscopy lists. The technique was comparatively In life the small bowel is roughly three metres easy for experienced endoscopists to in length: at necropsy (and during entero- learn. Forty two procedures were for diag- scopy) the organ may be stretched by nostic purposes. Eleven patients had 200-300%.1 Mobile and tortuously folded gastrointestinal bleeding where the source in the peritoneal cavity, it is little surprise was obscure, or where early investigations that endoscopy of this organ is technically had suggested a small bowel source: a challenging. Ten years after the first descrip- specific diagnosis was made in 450/0 of tions of non-operative enteroscopy2 it is these cases. -

Fehling...The Difference
FEHLING AORTIC PUNCHES INS TRUMENTS AORTENSTANZEN 11/1 FEHLING... ... THE DIFFERENCE INSTRUMENTS FOR THORACIC, CARDIAC AND VASCULAR SURGERY INSTRUMENTE FÜR THORAX-, HERZ- UND GEFÄSSCHIRURGIE FEHLING Hanauer Landstraße 7A · 63791 Karlstein/Germany · www.fehling-instruments.de INSTRUMENTS +49(0) 6188 - 9574.40 +49(0) 6188 - 9574.45 [email protected] FEHLING STERNAL RETRACTORS INSTRUMENTS STERNUMSPREIZER 11/2 CALAFIORE STERNAL RETAINER STERNUMOFFENHALTER 1 1 ⁄2 ⁄4 1 1 1 ⁄16 ⁄2 ⁄2 STERNUM BLADE SCREW NUT STERNUMBLATT FLAT WRENCH STORAGE TRAY MUTTER GABELSCHLÜSSEL LAGERUNGS- LEFT RIGHT SINGLE USE BEHÄLTER LINKS RECHTS MPA-5 MPC-1L MPC-1R NEONATAL 7 x 30 mm** MPB-1 7 x 30 mm* 7 x 30 mm* Ø 8 MPC-0P MPB-7L MPB-7R 10 x 18 mm* 10 x 18 mm* MPA-6 “PEDIATRIC“ PEDIATRIC 45 x 65 mm** MPB-2 MPA-2L MPA-2R Ø 12 10 x 50 mm* 10 x 50 mm* MPA-3L MPA-3R ADULT 15 x 70 mm* 15 x 70 mm* MPA-9 45 x 65 mm** MPC-0A Ø 16 “ADULT“ MPA-4L MPA-4R ADIPOSIS 20 x 100 mm* 20 x 100 mm* MPA-7 70 x 90 mm** MPB-3 Ø 16 MPB-5L MPB-5R 15 x 30 mm* 15 x 30 mm* MPA-8 MPC-0C OSTEOPOROSIS 95 x 115 mm** “CURVED“ MPB-6L MPB-6R Ø 16 20 x 30 mm* 20 x 30 mm* *blade size / Blattgröße **opening width / Öffnungsweite exemplary configuration exemplary configuration Beispielkonfiguration Beispielkonfiguration ADULT - ADIPOSIS OSTEOPOROSIS FEHLING RETRACTORS INSTRUMENTS SPREIZER 11/3 TILTING KIPPBAR FOR PARTIAL STERNOTOMY FÜR PARTIELLE STERNOTOMIE BLADE SIZE BLATTGRÖSSE SPREADING WIDTH 160 mm a x b SPREIZWEITE 100 mm 35 x 50 mm MRM-5 45 x 50 mm MRM-6 215 mm 1 ⁄3 MARJAN 2 ⁄3 SUPERFLEX SOFT -

Integra® Miltex® Female Patient Care Products
Integra® Miltex® Female Patient Care Products Limit uncertainty with our comprehensive selection of female patient care products Integra® Miltex® Female Patient Care Products Pessaries • Soft and pliable • Made of medical grade silicone* • Foldable for insertion and removal • Variety of shapes and sizes available Ring • Used to treat first or second-degree uterine proplase • Available with or without supporting membrane #0 #1 #2 #3 #4 #5 #6 #7 #8 #9 Size 1.75" 2.00" 2.25" 2.50" 2.75" 3.00" 3.25" 3.50" 3.75" 4.00" With Support 30-RS0 30-RS1 30-RS2 30-RS3 30-RS4 30-RS5 30-RS6 30-RS7 30-RS8 30-RS9 Without Support 30-R0 30-R1 30-R2 30-R3 30-R4 30-R5 30-R6 30-R7 30-R8 30-R9 Ring with Knob • Used to treat first or second-degree uterine proplase • May be used to treat stress urinary incontinence • Available with or without supporting membrane #0 #1 #2 #3 #4 #5 #6 #7 Size 1.75" 2.00" 2.25" 2.50" 2.75" 3.00" 3.25" 3.50" With Support 30-RKS0 30-RKS1 30-RKS2 30-RKS3 30-RKS4 30-RKS5 30-RKS6 30-RKS7 Without Support 30-RK0 30-RK1 30-RK2 30-RK3 30-RK4 30-RK5 30-RK6 30-RK7 Gellhorn • Used to treat second or third-degree uterine prolapse, cystocele or rectocele 1.5" • Drainage feature #0 #1 #2 #3 #4 #5 #6 #7 #8 Size 1.50" 1.75" 2.00" 2.25" 2.50" 2.75" 3.00" 3.25" 3.50" Standard Stem 30-GD0 30-GD1 30-GD2 30-GD3 30-GD4 30-GD5 30-GD6 30-GD7 30-GD8 Gellhorn Short-Stem • Used to treat second or third-degree uterine prolapse, cystocele or rectocele • Shorter stem may provide a better fit for some patients 1.1" • Drainage feature #0 #1 #2 #3 #4 #5 #6 #7 #8 Size -

8200 • Pipelle® Endometrial Suction Curette Directions for Use (English)
7. For histologic assessment of the specimen, the tip of the sheath should ® now be sectioned just proximal to its distal curette opening (Figure 4). 8200 • Pipelle Endometrial Suction Curette The piston should then be advanced within the sheath to express the specimen from the sectioned sheath into an appropriate transport medium Directions for Use (English) (Figure 5). Figure 4 Figure 5 DESCRIPTION The Pipelle® is a single-use, sterile, disposable, suction curette for obtaining a histologic biopsy of the uterine mucosal lining or sample extraction of uterine menstrual content for microscopic examination or culturing. The device consists of a clear, flexible, polypropylene sheath that is 26.5 cm (overall) 23.5 cm (effective) length with a 3.1 mm OD (Outside Diameter) and a 2.6 mm ID (Internal Diameter). The sheath is marked with colored, graduated markings from 4 cm to 10 cm, distance from the distal tip of the sheath to indicate the depth of insertion of the sheath into the uterus during use (see diagram below). 8. For bacterial culturing of the specimen, leave the piston in its fully withdrawn position and do not section the sheath at its distal curette A B 4 cm 8 cm C D EF opening. Immediately send the entire, intact instrument with the specimen 7 cm 10 cm captured within it to the laboratory with instructions to section the distal tip at a point at least 2 mm proximal to the distal curette opening and then to express the sample by use of the piston onto the appropriate culturing medium.