R J M E ASE EPORT Romanian Journal of C R Morphology & Embryology
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
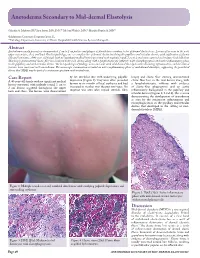
Anetoderma Secondary to Mid-Dermal Elastolysis
Anetoderma Secondary to Mid-dermal Elastolysis Gabriela A. Maloney, BS,* Jane James, MD, PhD,** Michael Welsch, MD,** Marylee Braniecki, MD** *Midwestern University, Downers Grove, IL **Pathology Department, University of Illinois Hospital & Health Sciences System, Chicago, IL Abstract Anetoderma usually presents as circumscribed, 1 cm to 2 cm patches and plaques of flaccid skin secondary to loss of dermal elastic tissue. Lesions often occur in the neck, upper extremities, chest, and back. On histopathology, one sees complete loss of dermal elastin involving the papillary and reticular dermis, with infiltration of plasma cells and histiocytes. A 40-year-old female with no significant medical history presented with multiple round, 1 cm to 2 cm lesions scattered on her upper back and chest. Skin biopsy demonstrated elastic-fiber loss localized to the mid-dermis along with a lymphohistiocytic infiltrate with elastophagocytosis and active inflammatory phase in the papillary and mid-reticular dermis. The histopathological findings were consistent with mid-dermal elastolysis with advancing inflammation, and the clinical features were consistent with anetoderma. The microscopic examination revealed an active inflammatory phase of mid-dermal elastolysis, supporting the postulated theory that MDE may be part of a continuous spectrum with anetoderma. Case Report by lax, wrinkled skin with underlying palpable biopsy and elastic-fiber staining demonstrated A 40-year-old female with no significant medical depression (Figure 1). They were often preceded elastic-fiber loss in the mid-dermis along with history presented with multiple round, 1 cm to by two to six months of local erythema and had a lymphohistiocytic infiltrate with evidence 2 cm lesions scattered throughout the upper increased in number over the past two years. -

De Barsy Syndrome
orphananesthesia Anesthesia recommendations for patients suffering from De Barsy syndrome Disease name: De Barsy syndrome ICD 10: Q87.7; OMIM 614438 Synonyms: DBS, De Barsy-Moens-Dierckx syndrome, Progeroid syndrome of De Barsy, Autosomal recessive cutis laxa Type 3 *With 2 gene subdivisions: ARCL3A: caused by a ALDH18A1 mutation ARCL3B: caused by a PYCR1 mutation DeBarsy syndrome is a rare clinical syndrome characterized by cutis laxa, ophthalmic opacification, skeletal malformations, as well as mental and growth retardation. This disease is genetically transmitted in an autosomal recessive fashion. Affected patients often require surgical correction of ophthalmic and orthopaedic abnormalities. This syndrome was first described by A.M. De Barsy in 1967 and less than 100 known cases are documented in the medical literature. Very little has been published on this rare disorder and only a single article has addressed anesthesia case outcomes and management strategies (Aponte, 2010). The diverse collection of clinical manifestations in De Barsy syndrome includes: intra-uterine growth retardation (IUGR), postnatal growth delay, motor delay, cognitive impairment, hypontonia, athetoid movements, malformations, microcephaly, wormian bones, large fontanelles, facial dysmorphism, cataracts, corneal clouding, thin/wrinkled skin, easy bruising, sparse hair, joint laxity, osteopenia, and inguinal hernias. Medicine in progress Perhaps new knowledge Every patient is unique Perhaps the diagnostic is wrong Find more information on the disease, its centres -

(12) Patent Application Publication (10) Pub. No.: US 2010/0210567 A1 Bevec (43) Pub
US 2010O2.10567A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2010/0210567 A1 Bevec (43) Pub. Date: Aug. 19, 2010 (54) USE OF ATUFTSINASATHERAPEUTIC Publication Classification AGENT (51) Int. Cl. A638/07 (2006.01) (76) Inventor: Dorian Bevec, Germering (DE) C07K 5/103 (2006.01) A6IP35/00 (2006.01) Correspondence Address: A6IPL/I6 (2006.01) WINSTEAD PC A6IP3L/20 (2006.01) i. 2O1 US (52) U.S. Cl. ........................................... 514/18: 530/330 9 (US) (57) ABSTRACT (21) Appl. No.: 12/677,311 The present invention is directed to the use of the peptide compound Thr-Lys-Pro-Arg-OH as a therapeutic agent for (22) PCT Filed: Sep. 9, 2008 the prophylaxis and/or treatment of cancer, autoimmune dis eases, fibrotic diseases, inflammatory diseases, neurodegen (86). PCT No.: PCT/EP2008/007470 erative diseases, infectious diseases, lung diseases, heart and vascular diseases and metabolic diseases. Moreover the S371 (c)(1), present invention relates to pharmaceutical compositions (2), (4) Date: Mar. 10, 2010 preferably inform of a lyophilisate or liquid buffersolution or artificial mother milk formulation or mother milk substitute (30) Foreign Application Priority Data containing the peptide Thr-Lys-Pro-Arg-OH optionally together with at least one pharmaceutically acceptable car Sep. 11, 2007 (EP) .................................. O7017754.8 rier, cryoprotectant, lyoprotectant, excipient and/or diluent. US 2010/0210567 A1 Aug. 19, 2010 USE OF ATUFTSNASATHERAPEUTIC ment of Hepatitis BVirus infection, diseases caused by Hepa AGENT titis B Virus infection, acute hepatitis, chronic hepatitis, full minant liver failure, liver cirrhosis, cancer associated with Hepatitis B Virus infection. 0001. The present invention is directed to the use of the Cancer, Tumors, Proliferative Diseases, Malignancies and peptide compound Thr-Lys-Pro-Arg-OH (Tuftsin) as a thera their Metastases peutic agent for the prophylaxis and/or treatment of cancer, 0008. -

With a Learning Disability; Guidance for General Practice
Improving identification of people with a learning disability: guidance for general practice NHS England and Improvement Publishing Approval Reference: 001030 Version 1 NHS England and NHS Improvement Contents Introduction .................................................................................... 2 Actions for practices ....................................................................... 4 Appendix 1: List of codes that indicate a learning disability ........... 7 Appendix 2: List of codes that may indicate a learning disability . 14 Appendix 3: List of outdated codes .............................................. 20 Appendix 4: Learning disability identification check-list ............... 22 1 | Contents Introduction 1. The NHS Long Term Plan1 commits to improve uptake of the existing annual health check in primary care for people aged over 14 years with a learning disability, so that at least 75% of those eligible have a learning disability health check each year. 2. There is also a need to increase the number of people receiving the annual seasonal flu vaccination, given the level of avoidable mortality associated with respiratory problems. 3. In 2017/18, only 44.6% of patients with a learning disability received a flu vaccination and only 55.1% of patients with a learning disability received an annual learning disability health check.2 4. In June 2019, NHS England and NHS Improvement announced a series of measures to improve coverage of annual health checks and flu vaccination for people with a learning disability. One of the commitments was to improve the quality of registers for people with a learning disability3. Clinical coding review 5. Most GP practices have developed a register of their patients known to have a learning disability. This has been developed from clinical diagnoses, from information gathered from learning disabilities teams and social services and has formed the basis of registers for people with learning disability developed for the Quality and Outcomes Framework (QOF). -

Dermatologists Cannot Be Lax About Acquired Cutis Laxa by Warren R
Dermatologists cannot be lax about acquired cutis laxa By Warren R. Heymann, MD Jan. 28, 2019 Acral localized acquired cutis laxa. Loose, redundant skin on the volar finger pads bilaterally that gave them a flat, rounded appearance. Credit: JAADFrom the time I was a medical student, I have always been fascinated by the “little old man” appearance of patient with cutis laxa (CL). In their excellent review, Berk et al note that CL is characterized by abnormal elastic fibers resulting in loose, redundant skin. Typically, skin in CL can easily be pulled and only slowly returns to its original position. Unlike some conditions in the differential diagnosis, notably Ehlers-Danlos syndrome(s) or pseudoxanthoma elasticum, easy bruising or abnormal scarring does not characterize CL. Redundant skin is often most noticeable on the neck, hands, and groin, but can also be seen on the face, creating a premature aging appearance. CL may be inherited or acquired (ACL). Inherited forms include autosomal dominant CL; autosomal recessive CL (ARCL)-I, -IIA, and -IIB; X-linked CL and other variants. Although all of the inherited forms of CL are rare, ARCL has most commonly been reported, particularly ARCL- II. Because of significant overlap among these types, precise clinical classification can be difficult. For the clinical differentiation of the variants and their genetic mutations, please refer to Berk et al (1). The etiology of CL involves abnormalities the elastic fiber network in skin and internal organs. Autosomal dominant CL has been associated with mutations of the elastin gene. X-linked CL is associated with gene mutations ATPA7 and abnormalities of copper transport. -
The Skin in Genetically-Controlled Metabolic Disorders P
Review Article J Med Genet: first published as 10.1136/jmg.10.2.101 on 1 June 1973. Downloaded from Journal of Medical Genetics (1973). 10, 101. The Skin in Genetically-controlled Metabolic Disorders P. C. H. NEWBOLD Department of Medicine, Cambridge University Medical School, Hills Road, Cambridge CB2 2QL Diseased nature oftentimes breaks forth however, it may lead to difficulty in assessing intelli- In strange eruptions.-Henry IV, part 1, III, i. gence, which is within normal range in 50% of The skin is now commonly accepted as a mirror patients. There is suggestive evidence of a re- of internal disease, but as with other looking-glasses, lationship between subnormal folate levels and low the evidence offered may be selected or ignored. intelligence (Carey et al, 1968), and studies have The epidermis is an interesting structure, especially shown increased turnover of folate coenzymes and rich in tyrosine, phenylalanine, tryptophan, and resulting folate depletion in these patients (Carey et histidine, when compared with the corium (Roth- al, 1968; Butterworth, Krumdieck, and Baugh, man, 1965). Tyrosinaemia and histidinaemia do 1971). There is also a high incidence of an organic not include cutaneous manifestations, but culture of brain syndrome following intracranial vascular skin fibroblasts is a helpful diagnostic tool for study- thromboses (Dunn, Perry, and Dolman, 1966), ing metabolic defects such as citrullinaemia, cysti- copyright. nosis, and maple-syrup urine disease (Scriver, 1969). Most of the conditions now to be described are rare, but if these metabolic diseases were com- mon, there could be no human race as we know it. Homocystinuria http://jmg.bmj.com/ This most informative anomaly was discovered during a study of mentally retarded patients in Ire- land (Carson and Neill, 1962). -

RIN2 Deficiency Results in Macrocephaly, Alopecia, Cutis Laxa
REPORT RIN2 Deficiency Results in Macrocephaly, Alopecia, Cutis Laxa, and Scoliosis: MACS Syndrome Lina Basel-Vanagaite,1,2,14 Ofer Sarig,4,14 Dov Hershkovitz,6,7 Dana Fuchs-Telem,2,4 Debora Rapaport,3 Andrea Gat,5 Gila Isman,4 Idit Shirazi,4 Mordechai Shohat,1,2 Claes D. Enk,10 Efrat Birk,2 Ju¨rgen Kohlhase,11 Uta Matysiak-Scholze,11 Idit Maya,1 Carlos Knopf,9 Anette Peffekoven,12 Hans-Christian Hennies,12 Reuven Bergman,8 Mia Horowitz,3 Akemi Ishida-Yamamoto,13 and Eli Sprecher2,4,6,* Inherited disorders of elastic tissue represent a complex and heterogeneous group of diseases, characterized often by sagging skin and occasionally by life-threatening visceral complications. In the present study, we report on an autosomal-recessive disorder that we have termed MACS syndrome (macrocephaly, alopecia, cutis laxa, and scoliosis). The disorder was mapped to chromosome 20p11.21-p11.23, and a homozygous frameshift mutation in RIN2 was found to segregate with the disease phenotype in a large consan- guineous kindred. The mutation identified results in decreased expression of RIN2, a ubiquitously expressed protein that interacts with Rab5 and is involved in the regulation of endocytic trafficking. RIN2 deficiency was found to be associated with paucity of dermal micro- fibrils and deficiency of fibulin-5, which may underlie the abnormal skin phenotype displayed by the patients. Disorders of elastic tissue often share common phenotypic shown to result in decreased secretion of elastin.9,10 In features, including redundant skin, hyperlaxity of joints, addition, -

Genetics of Lipedema: New Perspectives on Genetic Research and Molecular Diagnoses S
European Review for Medical and Pharmacological Sciences 2019; 23: 5581-5594 Genetics of lipedema: new perspectives on genetic research and molecular diagnoses S. PAOLACCI1, V. PRECONE2, F. ACQUAVIVA3, P. CHIURAZZI4,5, E. FULCHERI6,7, M. PINELLI3,8, F. BUFFELLI9,10, S. MICHELINI11, K.L. HERBST12, V. UNFER13, M. BERTELLI2; GENEOB PROJECT 1MAGI’S LAB, Rovereto (TN), Italy 2MAGI EUREGIO, Bolzano, Italy 3Department of Translational Medicine, Section of Pediatrics, Federico II University, Naples, Italy 4Istituto di Medicina Genomica, Fondazione A. Gemelli, Università Cattolica del Sacro Cuore, Rome, Italy 5UOC Genetica Medica, Fondazione Policlinico Universitario “A. Gemelli” IRCCS, Rome, Italy 6Fetal and Perinatal Pathology Unit, IRCCS Istituto Giannina Gaslini, Genoa, Italy 7Department of Integrated Surgical and Diagnostic Sciences, University of Genoa, Genoa, Italy 8Telethon Institute of Genetics and Medicine (TIGEM), Pozzuoli, Italy 9Fetal and Perinatal Pathology Unit, IRCCS Istituto Giannina Gaslini, Genoa, Italy 10Department of Neuroscience, Rehabilitation, Ophthalmology, Genetics and Maternal-Infantile Sciences, University of Genoa, Genoa, Italy 11Department of Vascular Rehabilitation, San Giovanni Battista Hospital, Rome, Italy 12Department of Medicine, University of Arizona, Tucson, AZ, USA 13Department of Developmental and Social Psychology, Faculty of Medicine and Psychology, Sapienza University of Rome, Rome, Italy Abstract. – OBJECTIVE: The aim of this quali- Introduction tative review is to provide an update on the cur- rent understanding of the genetic determinants of lipedema and to develop a genetic test to dif- Lipedema is an underdiagnosed chronic debil- ferentiate lipedema from other diagnoses. itating disease characterized by bruising and pain MATERIALS AND METHODS: An electronic and excess of subcutaneous adipose tissue of the search was conducted in MEDLINE, PubMed, and legs and/or arms in women during or after times Scopus for articles published in English up to of hormone change, especially in puberty1. -

WO 2015/048577 A2 April 2015 (02.04.2015) W P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2015/048577 A2 April 2015 (02.04.2015) W P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every A61K 48/00 (2006.01) kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, (21) International Application Number: BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, PCT/US20 14/057905 DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (22) International Filing Date: HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, 26 September 2014 (26.09.2014) KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, (25) Filing Language: English PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, (26) Publication Language: English SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: 61/883,925 27 September 2013 (27.09.2013) US (84) Designated States (unless otherwise indicated, for every 61/898,043 31 October 2013 (3 1. 10.2013) US kind of regional protection available): ARIPO (BW, GH, GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, (71) Applicant: EDITAS MEDICINE, INC. -

Metabolic Cutis Laxa Syndromes
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Springer - Publisher Connector J Inherit Metab Dis (2011) 34:907–916 DOI 10.1007/s10545-011-9305-9 CDG - AN UPDATE Metabolic cutis laxa syndromes Miski Mohamed & Dorus Kouwenberg & Thatjana Gardeitchik & Uwe Kornak & Ron A. Wevers & Eva Morava Received: 28 October 2010 /Revised: 11 February 2011 /Accepted: 17 February 2011 /Published online: 23 March 2011 # The Author(s) 2011. This article is published with open access at Springerlink.com Abstract Cutis laxa is a rare skin disorder characterized by Disorders of Glycosylation (CDG). Since then several wrinkled, redundant, inelastic and sagging skin due to inborn errors of metabolism with cutis laxa have been defective synthesis of elastic fibers and other proteins of the described with variable severity. These include P5CS, extracellular matrix. Wrinkled, inelastic skin occurs in ATP6V0A2-CDG and PYCR1 defects. In spite of the many cases as an acquired condition. Syndromic forms of evolving number of cutis laxa-related diseases a large part cutis laxa, however, are caused by diverse genetic defects, of the cases remain genetically unsolved. In metabolic cutis mostly coding for structural extracellular matrix proteins. laxa syndromes the clinical and laboratory features might Surprisingly a number of metabolic disorders have been partially overlap, however there are some distinct, discrim- also found to be associated with inherited cutis laxa. inative features. In this review on metabolic diseases Menkes disease was the first metabolic disease reported causing cutis laxa we offer a practical approach for the with old-looking, wrinkled skin. -

Acquired Cutis Laxa of Face with Multiple Myeloma
Letters to the Editor 6. Takayama K, Satoh T, Hayashi M, Yokozeki H. Psoriatic skin Serum β2‑microglobulin was increased. Bone marrow lesions induced by BCG vaccination. Acta Derm Venereol examination revealed 14% plasma cells. Based on 2008;88:621‑2. 7. Monacelli M. Koebner reaction in psoriasis. In: Farber EM, these findings, a diagnosis of early multiple myeloma Cox AJ, editors. Psoriasis: Proceedings of the International with nephrotic syndrome was made. She was started Symposium. Vol. 99. Stanford: Stanford University press; 1971. p. 104‑11. on a chemotherapy regimen consisting of thalidomide 8. Rambukkana A, Das PK, Witkamp L, Yong S, Meinardi MM, (100 mg/day), oral cyclophosphamide (150 mg/day) Bos JD. Antibodies to mycobacterial 65‑kDa heat shock protein both for the first five days in every month with weekly and other immunodominantantigens in patients with psoriasis. J Invest Dermatol 1993;100:87‑92. intravenous dexamethasone (40 mg/week) along with loop diuretics. In addition, the patient was prescribed Access this article online bortezomib which was discontinued by the patient after two months. Following one year of treatment, the Quick Response Code: Website: www.ijdvl.com anasarca subsided leaving behind loose wrinkled skin all over the body. Over the next one year, the skin over the DOI: 10.4103/0378-6323.140309 extremities and the abdomen reverted back to normal; however, loosening of skin persisted over the face. PMID: ***** Examination revealed a healthy‑appearing woman, who appeared older than her stated age [Figure 1]. She Acquired cutis laxa of face with had loose, sagging skin on the face and neck giving her a hound‑dog facies [Figure 2]. -

Discriminative Features in Three Autosomal Recessive Cutis Laxa Syndromes: Cutis Laxa IIA, Cutis Laxa IIB, and Geroderma Osteoplastica
International Journal of Molecular Sciences Review Discriminative Features in Three Autosomal Recessive Cutis Laxa Syndromes: Cutis Laxa IIA, Cutis Laxa IIB, and Geroderma Osteoplastica Ariana Kariminejad 1,*, Fariba Afroozan 1, Bita Bozorgmehr 1, Alireza Ghanadan 2,3, Susan Akbaroghli 4, Hamid Reza Khorram Khorshid 5, Faezeh Mojahedi 6, Aria Setoodeh 7, Abigail Loh 8, Yu Xuan Tan 8, Nathalie Escande-Beillard 8, Fransiska Malfait 9, Bruno Reversade 8, Thatjana Gardeitchik 10 and Eva Morava 10,11 1 Kariminejad-Najmabadi Pathology & Genetics Center, #2, 4th Street, Hasan Seyf Street, Sanat Square, Tehran 14667-13713, Iran; [email protected] (F.A.); [email protected] (B.B.) 2 Department of Dermatopathology, Razi Dermatology Hospital, Tehran University of Medical Sciences, Tehran 14167-53955, Iran; [email protected] 3 Department of Pathology, Cancer Institute, Imam Khomeini Hospital Complex, Tehran University of Medical Sciences, Tehran 14197-33141, Iran 4 Clinical Genetics Division, Mofid Children’s Hospital, Faculty of Medicine, Shahid Beheshti University of Medical Sciences, Tehran 15514-15468, Iran; [email protected] 5 Genetic Research Centre, University of Social Welfare and Rehabilitation Sciences, Tehran 19857-13834, Iran; [email protected] 6 Mashhad Medical Genetic Counseling Center, Social Welfare and Rehabilitation Organization, Mashhad 91767-61999, Iran; [email protected] 7 Division of Pediatric Endocrinology and Inherited Metabolic Disorders, Department of Pediatrics, Tehran University of Medical Sciences, Tehran