Dry Tortugas Coastal Marine Ecosystem
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

FWC Division of Law Enforcement South Region
FWC Division of Law Enforcement South Region – Bravo South Region B Comprised of: • Major Alfredo Escanio • Captain Patrick Langley (Key West to Marathon) – Lieutenants Roy Payne, George Cabanas, Ryan Smith, Josh Peters (Sanctuary), Kim Dipre • Captain David Dipre (Marathon to Dade County) – Lieutenants Elizabeth Riesz, David McDaniel, David Robison, Al Maza • Pilot – Officer Daniel Willman • Investigators – Carlo Morato, John Brown, Jeremy Munkelt, Bryan Fugate, Racquel Daniels • 33 Officers • Erik Steinmetz • Seth Wingard • Wade Hefner • Oliver Adams • William Burns • John Conlin • Janette Costoya • Andy Cox • Bret Swenson • Robb Mitchell • Rewa DeBrule • James Johnson • Robert Dube • Kyle Mason • Michael Mattson • Michael Bulger • Danielle Bogue • Steve Golden • Christopher Mattson • Steve Dion • Michael McKay • Jose Lopez • Scott Larosa • Jason Richards • Ed Maldonado • Adam Garrison • Jason Rafter • Marty Messier • Sebastian Dri • Raul Pena-Lopez • Douglas Krieger • Glen Way • Clayton Wagner NOAA Offshore Vessel Peter Gladding 2 NOAA near shore Patrol Vessels FWC Sanctuary Officers State Law Enforcement Authority: F. S. 379.1025 – Powers of the Commission F. S. 379.336 – Citizens with violations outside of state boundaries F. S. 372.3311 – Police Power of the Commission F. S. 910.006 – State Special Maritime Jurisdiction Federal Law Enforcement Authority: U.S. Department of Commerce - National Marine Fisheries Service U.S. Department of the Interior - U.S. Fish & Wildlife Service U.S. Department of the Treasury - U.S. Customs Service -

MSRP Appendix A
APPENDIX A: RECOVERY TEAM MEMBERS Multi-Species Recovery Plan for South Florida Appendix A. Names appearing in bold print denote those who authored or prepared Appointed Recovery various components of the recovery plan. Team Members Ralph Adams Geoffrey Babb Florida Atlantic University The Nature Conservancy Biological Sciences 222 South Westmonte Drive, Suite 300 Boca Raton, Florida 33431 Altimonte Springs, Florida 32714-4236 Ross Alliston Alice Bard Monroe County, Environmental Florida Department of Environmental Resource Director Protection 2798 Overseas Hwy Florida Park Service, District 3 Marathon , Florida 33050 1549 State Park Drive Clermont, Florida 34711 Ken Alvarez Florida Department of Enviromental Bob Barron Protection U.S. Army Corps of Engineers Florida Park Service, 1843 South Trail Regulatory Division Osprey, Florida 34229 P.O. Box 4970 Jacksonville, Florida 32232-0019 Loran Anderson Florida State University Oron L. “Sonny” Bass Department of Biological Science National Park Service Tallahassee, Florida 32306-2043 Everglades National Park 40001 State Road 9336 Tom Armentano Homestead, Florida 33034-6733 National Park Service Everglades National Park Steven Beissinger 40001 State Road 9336 Yale University - School of Homestead, Florida 33034-6733 Forestry & Environmental Studies Sage Hall, 205 Prospect Street David Arnold New Haven, Connecticut 06511 Florida Department of Environmental Protection Rob Bennetts 3900 Commonwealth Boulevard P.O. Box 502 Tallahassee, Florida 32399-3000 West Glacier, Montana 59936 Daniel F. Austin Michael Bentzien Florida Atlantic University U.S. Fish and Wildlife Service Biological Sciences Jacksonville Field Office 777 Glades Road 6620 Southpoint Drive South, Suite 310 Boca Raton, Florida 33431 Jacksonville, Florida 32216-0912 David Auth Nancy Bissett University of Florida The Natives Florida Museum of Natural History 2929 J.B. -

Keys Sanctuary 25 Years of Marine Preservation National Parks Turn 100 Offbeat Keys Names Florida Keys Sunsets
Keys TravelerThe Magazine Keys Sanctuary 25 Years of Marine Preservation National Parks Turn 100 Offbeat Keys Names Florida Keys Sunsets fla-keys.com Decompresssing at Bahia Honda State Park near Big Pine Key in the Lower Florida Keys. ANDY NEWMAN MARIA NEWMAN Keys Traveler 12 The Magazine Editor Andy Newman Managing Editor 8 4 Carol Shaughnessy ROB O’NEAL ROB Copy Editor Buck Banks Writers Julie Botteri We do! Briana Ciraulo Chloe Lykes TIM GROLLIMUND “Keys Traveler” is published by the Monroe County Tourist Development Contents Council, the official visitor marketing agency for the Florida Keys & Key West. 4 Sanctuary Protects Keys Marine Resources Director 8 Outdoor Art Enriches the Florida Keys Harold Wheeler 9 Epic Keys: Kiteboarding and Wakeboarding Director of Sales Stacey Mitchell 10 That Florida Keys Sunset! Florida Keys & Key West 12 Keys National Parks Join Centennial Celebration Visitor Information www.fla-keys.com 14 Florida Bay is a Must-Do Angling Experience www.fla-keys.co.uk 16 Race Over Water During Key Largo Bridge Run www.fla-keys.de www.fla-keys.it 17 What’s in a Name? In Marathon, Plenty! www.fla-keys.ie 18 Visit Indian and Lignumvitae Keys Splash or Relax at Keys Beaches www.fla-keys.fr New Arts District Enlivens Key West ach of the Florida Keys’ regions, from Key Largo Bahia Honda State Park, located in the Lower Keys www.fla-keys.nl www.fla-keys.be Stroll Back in Time at Crane Point to Key West, features sandy beaches for relaxing, between MMs 36 and 37. The beaches of Bahia Honda Toll-Free in the U.S. -

Fkeys-CMP.Pdf
Florida KEYS Scenic Highway corridor management plan Submitted to Florida Department of Transportation, District Six Scenic Highways Coordinator 602 South Miami Avenue Miami, FL 33130 Submitted by The Florida Keys Scenic Highway CAG June Helbling and Kathy Toribio, Co-Chairs c/o Clean Florida Keys, Inc. PO Box 1528 Key West, FL 33041-1528 Prepared by The Florida Keys Scenic Highway CAG Peggy Fowler, Planning Consultant Patricia Fontova, Graphic Designer Carter and Burgess, Inc., Planning Consultants May, 2001 This document was prepared in part with funding from the Florida Department of Transportation. This document is formatted for 2-sided printing. Some pages were left intentionally blank for that reason. Table of Contents Chapter 1: INTRODUCTION .....................................................1 Chapter 2: CORRIDOR VISION ..................................................5 Chapter 3: CORRIDOR STORY ..................................................7 Chapter 4: DESIGNATION CRITERIA .......................................13 Chapter 5: BACKGROUND CONDITONS ANALYSIS ...............27 Chapter 6: RELATIONSHIP TO COMPREHENSIVE PLAN .......59 Chapter 7: PROTECTION TECHNIQUES................................ .63 Chapter 8: COMMUNITY PARTICIPATION ..............................69 Chapter 9: PARTNERSHIPS AND AGREEMENTS.................... .79 Chapter 10: FUNDING AND PROMOTION ...............................85 Chapter 11: GOALS, OBJECTIVES AND STRATEGIES ................93 Chapter 12: ACTION PLAN .........................................................97 -

Integrated Conceptual Ecosystem Model Development for the Southeast Florida Coastal Marine Ecosystem
NOAA Technical Memorandum OAR-AOML-103/NOS-NCCOS-163 Integrated Conceptual Ecosystem Model Development for the Southeast Florida Coastal Marine Ecosystem MARine Estuarine goal Setting (MARES) for South Florida Produced by the National Oceanic and Atmospheric Administration in Cooperation with Federal, State, Local, Academic, Industry Partners, and Non-Government Organizations June 2013 U.S. Department of Commerce | National Oceanic and Atmospheric Administration Suggested Citation Entire document: Nuttle, W.K., and P.J. Fletcher (eds.). 2013. Integrated conceptual ecosystem model development for the Southeast Florida coastal marine ecosystem. NOAA Technical Memorandum, OAR‑AOML‑103 and NOS‑NCCOS‑163. Miami, Florida. 125 pp. For appendices (as an example): Ault, J.S., J. Browder, and W.K. Nuttle. 2013. Fish and shellfish. In Integrated Conceptual Ecosystem Model Development for the Southeast Florida Coastal Marine Ecosystem, W.K. Nuttle and P.J. Fletcher (eds.). NOAA Technical Memorandum, OAR‑AOML‑103 and NOS‑NCCOS‑163. Miami, Florida. 53‑62. Acknowledgments This paper is a result of research under the MARine and Estuarine goal Setting (MARES) for South Florida Project funded by the National Oceanic and Atmospheric Administration Center for Sponsored Coastal Ocean Research (Coastal Ocean Program), under award NA08OAR4320889 to the University of Miami, NA09NOS4780224 to Nova Southeastern University, NA09NOS4780225 to the University of Massachusetts Amherst, NA09NOS4780226 to the National Audubon Society, NA‑ 09NOS4780227 to Florida Gulf Coast University, NA09NOS4780228 to Florida International Uni‑ versity, and to the NOAA Atlantic Oceanographic and Meteorological Laboratory. We thank Gail Derr of NOAA’s Atlantic Oceanographic and Meteorological Laboratory for her support in developing this technical memorandum. Disclaimer NOAA does not approve, recommend, or endorse any proprietary pr oduct or material mentioned in this document. -

Arts and Cultural Events Calendar
the art oF GivinG o Individual $50 o Business non-profit $250 o Contributor $100 o Business Partner $375 arts and cultural o Advocate $250 o Corporate Partner $1,000 o Supporter $500 o Corporate Sponsor $5,000 events calendar o Patron $1,000 o I would like to Volunteer o Benefactor $5,000 o I am an artist Name ________________________________________________ Business / Organization __________________________________ Address _______________________________________________ _______________________________________________ keys Florida KeyS Email ________________________________________________ Phone ____________________ Cell ________________________ CounCil oF the Make checks payable to FKCA. Amount enclosed $ _____________ arts Credit Card No. __________________________ Exp._____________ For your membership you will receive email updates, Calls to Artists, newsletters & advance notice of special events & discounts on workshops. Additional gifts are tax deductible as allowed by law. Florida KeyS CounCil oF the artS 1100 Simonton Street, Key West, Florida 33040 •April 305-295-4369 •May www.keysarts.com e-mail: [email protected] Visit www.keysarts.com for listings of theater, music, film, dance, lectures, meetings, •June classes, workshops, cultural activities and calls to artists. This Week’s Events The mission of the Florida Keys Council of the Arts is to advance the creative 2013 development and promotion of the arts in our cultural community by providing excellence in leadership, advocacy, education and financial support for artists, cultural organizations and citizens of Monroe county. We Support • We ConneCt • We promote • We Give all the KeyS This publication is sponsored by the Florida Keys Council of the Arts, all the artS the Monroe County Tourist Development Council, The State of Florida, Department of State, Division of Cultural Affairs, all the time the Florida Council on Arts and Culture, National Endowment for the Arts and our wonderful advertisers and donors. -

Mile Marker 0-65 (Lower Keys)
Key to Map: Map is not to scale Existing Florida Keys Overseas Heritage Trail Aquatic Preserves or Alternate Path Overseas Paddling Trail U.S. 1 Point of Interest U.S. Highway 1 TO MIAMI Kayak/Canoe Launch Site CARD SOUND RD Additional Paths and Lanes TO N KEY LARGO Chamber of Commerce (Future) Trailhead or Rest Area Information Center Key Largo Dagny Johnson Trailhead Mangroves Key Largo Hammock Historic Bridge-Fishing Botanical State Park Islands Historic Bridge Garden Cove MM Mile Marker Rattlesnake Key MM 105 Florida Department of Environmental Protection, Office of Greenways & Trails Florida Keys Overseas Heritage Trail Office: (305) 853-3571 Key Largo Adams Waterway FloridaGreenwaysAndTrails.com El Radabob Key John Pennekamp Coral Reef State Park MM 100 Swash Friendship Park Keys Key Largo Community Park Florida Keys Community of Key Largo FLORIDA BAY MM 95 Rodriguez Key Sunset Park Dove Key Overseas Heritage Trail Town of Tavernier Harry Harris Park Burton Drive/Bicycle Lane MM 90 Tavernier Key Plantation Key Tavernier Creek Lignumvitae Key Aquatic Preserve Founders Park ATLANTIC OCEAN Windley Key Fossil Reef Geological State Park MM 85 Snake Creek Long Key Historic Bridge TO UPPER Islamorada, Village of Islands Whale Harbor Channel GULF OF MEXICO KEYS Tom's Harbor Cut Historic Bridge Wayside Rest Area Upper Matecumbe Key Tom's Harbor Channel Historic Bridge MM 80 Dolphin Research Center Lignumvitae Key Botanical State Park Tea Table Key Relief Channel Grassy Key MM 60 Conch Keys Tea Table Channel Grassy Key Rest Area Indian Key -
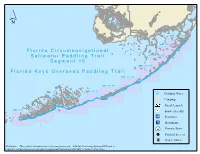
Segment 15 Map Book
´ M M 1 0 4 - 8 9 M M 9 8 - 8 9 Florida Circumnavigational Saltwater Paddling Trail M M 9 1 - 8 1 S e g m e n t 1 5 M M 8 2 - 7 0 Florida Keys Overseas Paddling Trail M M 7 1 - 5 9 M M 6 0 - 4 8 M M 5 0 - 3 9 po Drinking Water M M 2 7 - 1 7 M M 3 9 - 2 7 M M 2 7 - 1 7 t[ Camping M M 1 7 - 7 Kayak Launch M M 8 - 0 M M 8 - 0 Shower Facility I* Restroom I9 Restaurant ²· Grocery Store !e Point of Interest l Hotel / Motel Disclaimer: This guide is intended as an aid to navigation only. A Gobal Positioning System (GPS) unit is required, and persons are encouraged to supplement these maps with NOAA charts or other maps. li d[ li 3 Boggy Key 12 6 Mile Markers 104-96 Bush Point 3 N:25.1252 | W: -80.4054 op [ MM 104 A I* t li Porjoe Key ´ 3 Largo Sound MM 103 6 3 li Whaleback Key A J3 ohn PenneKamp State ParK 6 3 d[ MM 102 li El Radabob Key Swash Keys FL Keys National MM 101 Marine Sanctuary li 6 [ Key Largo d MONROE 6 Shell Key li MM 100 18 d[ 3 Pelican Key MM 99 3 6 li 3 Butternut Key 3 John Pennekamp d[ MM 98 li Coral Reef State Park 6 3 3 MM 97 6 12 6 6 li 6 MM 96 Pigeon Key Verdera Beaclih Rodriguez Key li 0 1 2 4 Miles Sunset Point 12 3 Dove Key li li Mile Markers 98-89 Butternut Key 3 MM 98 A N:25.0242 | W: -80.4943 op I* li 12 3 3 d[ 3 MM 97 ´ 6 li John Pennekamp Bottle Key Coral Reef State Park 12 Stake Key MM 96 6 Pigeon Key li d[ Rodriguez Key 6 Low Key 3 d[ Wild Bird Center MM 95 !e !e li Dove Key MONROd[E 6 12 Tavernier Key MM 94 12 3 li Island Bay Motel !e Dove Creek Conservation Area N: 25.0165 I W: -80.5133 MM 93 3 li d[A Harry -

Florida Keys Overseas Heritage Trail
9/15 FloridaStateParks.org/floridakeys FloridaGreenwaysAndTrails.com. (305) 853-3571 (305) 3 La Croix Court, Key Largo, FL 33037 FL Largo, Key Court, Croix La 3 of the Florida Keys Overseas Paddling Trail, visit Trail, Paddling Overseas Keys Florida the of Florida Keys Overseas Heritage Trail Heritage Overseas Keys Florida of Greenways & Trails. For maps and descriptions and maps For Trails. & Greenways of Division of Recreation and Parks and Recreation of Division Paddling Trail, a project coordinated by the Office the by coordinated project a Trail, Paddling Florida Department of Environmental Protection Environmental of Department Florida 1500-mile Florida Circumnavigational Saltwater Circumnavigational Florida 1500-mile the environment. The Trail is a segment of the of segment a is Trail The environment. the recreational opportunities that minimally impact minimally that opportunities recreational and ecology of the Keys while providing while Keys the of ecology and The “blueway” introduces visitors to the history the to visitors introduces “blueway” The Overseas Heritage Trail. Trail. Heritage Overseas the growing sport of kayak and canoe touring. canoe and kayak of sport growing the of Recreation and Parks as part of the Florida Keys Florida the of part as Parks and Recreation of a paddling destination for those involved in involved those for destination paddling a Department of Environmental Protection’s Division Protection’s Environmental of Department The Florida Keys Overseas Paddling Trail offers Trail Paddling Overseas Keys Florida The Historic Places and are managed by the Florida the by managed are and Places Historic have been listed on the National Register of Register National the on listed been have Trail all of the remaining 23 Flagler Railroad Bridges Railroad Flagler 23 remaining the of all Florida Keys Overseas Paddling Paddling Overseas Keys Florida into an economically viable destination. -

Backcountryplan.Pdf
EXHIBIT A MANAGEMENT AGREEMENT for BACKCOUNTRY PORTIONS of KEY WEST NATIONAL WILDLIFE REFUGE GREAT WHITE HERON NATIONAL WILDLIFE REFUGE and NATIONAL KEY DEER REFUGE Monroe County, Florida SEPTEMBER 1992 UNITED STATES DEPARTMENT OF THE INTERIOR FISH AND WILDLIFE SERVICE 75 SPRING STREET, S.W. ATLANTA, GEORGIA 30303 and STATE OF FLORIDA DEPARTMENT OF NATURAL RESOURCES TALLAHASSEE, FLORIDA TABLE OF CONTENTS INTRODUCTION PART I - BACKGROUND P a g e 1. Purposes for Establishment of the National Wildlife Refuges in the Lower Florida Keys 2. Management Authority 3. Environment 4. Traditional Uses 5. Resources A. General Habitat Characteristics B. Endangered and Threatened Species 6. Administration 7. Land Status 8. Management Activities (1986 - present) 9. Agreements and Permits PART II - RESOURCE PROBLEMS 1. Literature Review: Human disturbance of wildlife A. Overview B. Potential Effects of Human Disturbance on Birds C. Colonially Nesting Waterbirds D. Raptors 2. Special Considerations A. Bald Eagles B. Ospreys C. Magnificent Frigatebirds D. Mangrove Islands 3. Personal Watercraft (jet skis) A. Definitions B. Background C. Numbers D. Distribution E. Problems 4. Airboats 5. Water Skiing 6. Commercial Use 7. Law Enforcement Problems 8. Conflicts Between User Groups 9. Loss of Wilderness Values ii PART III - REFUGE OBJECTIVES AND MANAGEMENT STRATEGY P a g e 1. Refuge Objectives A. Highest Priority B. High Priority C. Moderate Priority 2. Management Strategy PART IV - MANAGEMENT ACTIONS 1. Idle Speed, No Motor, and No Access Buffer Zones A. Overview B. Resources Available/Current Program C. Proposed Management a. Definitions b. Organization c. Signage d. Key West National Wildlife Refuge e. Great White Heron National Wildlife Refuge 2. -

Lignumvitae Key Botanical State Park
Lignumvitae Key Botanical State Park APPROVED Unit Management Plan STATE OF FLORIDA Department of Environmental Protection Division of Recreation and Parks April 23, 2012 TABLE OF CONTENTS INTRODUCTION ..........................................................................................................1 PURPOSE AND SIGNIFICANCE OF THE PARK.....................................................1 PURPOSE AND SCOPE OF THE PLAN .....................................................................2 MANAGEMENT PROGRAM OVERVIEW................................................................8 Management Authority and Responsibility...................................................................8 Park Management Goals ..............................................................................................9 Management Coordination...........................................................................................9 Public Participation....................................................................................................10 Other Designations ....................................................................................................10 RESOURCE MANAGEMENT COMPONENT INTRODUCTION ........................................................................................................11 RESOURCE DESCRIPTION AND ASSESSMENT ..................................................12 Natural Resources......................................................................................................12 Topography...........................................................................................................12 -

MCTG-2753 ENGLISH Conch Brochure
For More Information KEY LARGO CHAMBER OF COMMERCE (305) The Florida Keys & Key West. Come as you are. FLORIDA KEYS VISITOR CENTER (305) 451-1414 [email protected]/[email protected] Milemarker 106, Overseas Hwy. Key Largo, FL 3451-14143037, U.S. ISLAMORADA CHAMBER OF COMMERCE 305-664-4503305-664-4503/[email protected]@islamoradachamber.org Compose your vacation in any Key. Milemarker 82.6, Overseas Hwy., P.O. Box 915 Islamorada, FL 33036, U.S. MARATHON CHAMBER OF COMMERCE ((305)305 )743-5417 743-5417/[email protected]@floridakeysmarathon.com Milemarker 53, Overseas Hwy. Marathon, FL 33050, U.S. LOWER KEYS CHAMBER OF COMMERCE (305) 872-2411/[email protected] (305) 872-2411 [email protected] KEY LARGO ISLAMORADA MARATHON LOWER KEYS KEY WEST Milemarker 31, Overseas Hwy. Big Pine Key, FL 33040, U.S. Sport Fishing Your Hometown in the KEY WEST CHAMBER OF COMMERCE ((305)305 )294-2587 [email protected]/[email protected] Dive Capital of the World. A Natural Escape. The Uncommon Place. 510 Greene Street, Key West, FL 33040, U.S. Capital of the World. Heart of the Keys. FLORIDA KEYS & KEY WEST TOURIST DEVELOPMENT COUNCIL (305) 296-1552/1-800-FLA-KEYS P.O. Box 866, Key West, FL 33041, U.S. Directions BY AIR: TheThe KeyKey WestWest IInternationalnternationa lAirport Airpor hast ha scommercial commerci aservicel servic ande and is iserveds serve dby b y major airlines. Bmajor airlines.oth the Key We Bothst and the M aKeyrat hWeston a iandrpo rMarathonts have ch airportsarter se rhavevice, chartergenera lservice, aviatio n and rental service.