1999 AAZV Proceedings.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lindane Lotion USP, 1% RX Only WARNINGS
Lindane Lotion USP, 1% RX Only WARNINGS: Lindane Lotion should only be used in patients who cannot tolerate or have failed first-line treatment with safer medications for the treatment of scabies. (See INDICATIONS AND USAGE.) Neurologic Toxicity Seizures and deaths have been reported following Lindane Lotion use with repeat or prolonged application, but also in rare cases following a single application used according to directions. Lindane lotion should be used with caution for infants, children, the elderly, and individuals with other skin conditions (e.g, atopic dermatitis, psoriasis) and in those who weigh < 110 lbs (50 kg) as they may be at risk of serious neurotoxicity. Contraindications Lindane Lotion is contraindicated in premature infants and individuals with known uncontrolled seizure disorders. Proper Use Instruct patients on the proper use of Lindane Lotion, the amount to apply, how long to leave it on, and avoiding re-treatment. Inform patients that itching occurs after the successful killing of scabies and is not necessarily an indication for re-treatment with Lindane Lotion. (See DOSAGE AND ADMINISTRATION.) DESCRIPTION Lindane Lotion USP, 1%, is an ectoparasiticide and ovicide. In addition to the active ingredient, lindane, it contains glycerol monostearate, cetyl alcohol, stearic acid, trolamine, carrageenan, 2- amino-2-methyl-1-propanol, methylparaben, butylparaben, perfume and water to form a lotion. Lindane is the gamma isomer of 1,2,3,4,5,6-hexachlorocyclohexane having the following structural formula: C6H6Cl6 M.W. 290.83 CLINICAL PHARMACOLOGY Lindane Lotion USP, 1%, is an ectoparasiticide and ovicide effective against Sarcoptes scabiei (scabies). Lindane exerts its parasiticidal action by being directly absorbed into the parasites and 1 their ova. -

Revised Use-Function Classification (2007)
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY IPCS INTOX Data Management System (INTOX DMS) Revised Use-Function Classification (2007) The Use-Function Classification is used in two places in the INTOX Data Management System: the Communication Record and the Agent/Product Record. The two records are linked: if there is an agent record for a Centre Agent that is the subject of a call, the appropriate Intended Use-Function can be selected automatically in the Communication Record. The Use-Function Classification is used when generating reports, both standard and customized, and for searching the case and agent databases. In particular, INTOX standard reports use the top level headings of the Intended Use-Functions that were selected for Centre Agents in the Communication Record (e.g. if an agent was classified as an Analgesic for Human Use in the Communication Record, it would be logged as a Pharmaceutical for Human Use in the report). The Use-Function classification is very important for ensuring harmonized data collection. In version 4.4 of the software, 5 new additions were made to the top levels of the classification provided with the system for the classification of organisms (items XIV to XVIII). This is a 'convenience' classification to facilitate searching of the Communications database. A taxonomic classification for organisms is provided within the INTOX DMS Agent Explorer. In May/June 2006 INTOX users were surveyed to find out whether they had made any changes to the Use-Function Classification. These changes were then discussed at the 4th and 5th Meetings of INTOX Users. Version 4.5 of the INTOX DMS includes the revised pesticides classification (shown in full below). -

Antibiotics May Eradicate Gastrointestinal Immunodeficiency.8
1122 Letters to the Editor J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.54.12.1122 on 1 December 1991. Downloaded from sitivity.? We present a case of OMM without pseudobulbar palsy. The association of vitro.8 Whipple's disease is associated with symptoms of Whipple's disease and with supranuclear gaze paresis with oculomas- immunodeficiency.8 Thus intestinal wall negative peroral jejunoileal biopsies, which ticatory myorhythmia, however, seems to be macrophages are ineffective in phagocytosing indicates the usefulness of laparotomy for pathognomonic of the effects of Whipple's intracellular gram positive bacilli, resulting in jejunoileal biopsies as an alternative to brain disease on the CNS.'2 This led us to perform inability to eliminate chronic infection.9 This biopsy to confirm Whipple's disease. surgical jejunal and mesenteric lymph node suggests that Whipple's disease may be con- A 47 year old woman was admitted in May biopsies rather than a brain biopsy despite sidered as a disease of macrophages.8 The 1988 in a depressed state. In June 1987 she negative endoscopic and numerous peroral periventricular and periaqueductal distribu- had noted progressive visual disturbance. distal jejunal and ileal biopsies. The nor- tion of the CNS involvement in Whipple's Rhythmic elevations of her right upper lip mality ofthe CT and MRI scans also suppor- disease consists of macrophagic infiltration appeared, and later, paroxysmal hypersomnia ted this decision. and subependymal nodules. Such a and considerable weight gain (10 kg). A nine The possibility of brain involvement with- "tumoural" involvement may explain why month course oftreatment for depression was out systemic manifestation in Whipple's dis- antibiotics with good BBB diffusion are not ineffective and her symptoms and signs ease should be kept in mind. -
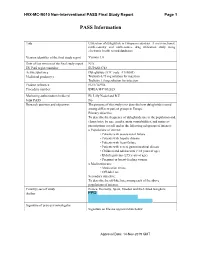
PASS Information
H9X-MC-B010 Non-interventional PASS Final Study Report Page 1 PASS Information Title Utilisation of dulaglutide in European countries: A cross-sectional, multi-country and multi-source drug utilisation study using electronic health record databases Version identifier of the final study report Version 1.0 Date of last version of the final study report N/A EU PAS register number EUPAS13783 Active substance Dulaglutide (ATC code: A10BJ05) Medicinal product(s): Trulicity 0.75 mg solution for injection Trulicity 1.5 mg solution for injection Product reference: EU/1/14/956 Procedure number: EMEA/H/C/002825 Marketing authorisation holder(s) Eli Lilly Nederland B.V. Joint PASS No Research question and objectives The purpose of this study is to describe how dulaglutide is used among different patient groups in Europe. Primary objective: To describe the frequency of dulaglutide use in the population and characterise by age, gender, main comorbidities, and main co- prescriptions overall and in the following subgroups of interest: o Populations of interest: • Patients with severe renal failure • Patients with hepatic disease • Patients with heart failure • Patients with severe gastrointestinal disease • Children and adolescents (<18 years of age) • Elderly patients (≥75 years of age) • Pregnant or breast-feeding women o Medication use: • Medication errors • Off-label use Secondary objective: To describe the off-label use among each of the above populations of interest. Country(-ies) of study France, Germany, Spain, Sweden and the United Kingdom Author PPD Signature of principal investigator Signature on file/see approval date below Approval Date: 14-Nov-2019 GMT H9X-MC-B010 Non-interventional PASS Final Study Report Page 2 Marketing Authorisation Holder Marketing authorisation holder (MAH) Eli Lilly Nederland B.V. -

A Saprolegnia Parasitica Challenge System for Rainbow Trout: Assessment of Pyceze As an Anti-Fungal Agent for Both Fish and Ova
DISEASES OF AQUATIC ORGANISMS Published May 12 Dis Aquat Org l A Saprolegnia parasitica challenge system for rainbow trout: assessment of Pyceze as an anti-fungal agent for both fish and ova T. G.Pottinger*, J. G. Day NERC Institute of Freshwater Ecology, Windermere Laboratory, Far Sawrey, Ambleside, Cumbria, LA22 OLP, United Kingdom ABSTRACT: A reproducible Saprolegnia parasitica spore delivery system was developed and demon- strated to be effective in providing a sustained spore challenge for up to 10 d. Treatment of rainbow trout with slow-release intraperitoneal implants containing cortisol resulted in chronically elevated blood cortisol levels and rendered the fish susceptible to infection by S. parasitica when exposed to the spore challenge. Sham-implanted fish were not susceptible to infect~on.Bronopol (2-bromo-2-nitro- propane-1,3-dol), formulated as Pyceze, was effective in protecting predisposed fish from infection by S. parasitica when administered as a daily bathlflush treatment at concentrations of 15 mg I-' and greater. Pyceze was also demonstrated to protect fertilised rainbow trout ova from S. parasitica chal- lenge when administered as a daily bath/flush treatment at concentrations of between 30 and 100 mg 1-l. Pyceze appears to qualify as a safe and effective replacement for malachite green and formalin in the prevention of fungal infections in the aquaculture environment. KEY WORDS: Saprolegnia . Fungal infection . Salmonid . Bronopol - Pyceze . Cortisol INTRODUCTION chite green in combating mycotic infections of fish and fish eggs, but is safer for the operator, the fish, and Mycotic infections of farmed fish, primarily by water the environment. Among the alternative compounds moulds or pseudofungi of the genus Saprolegnia, rep- tested for antifungal activity are sodium chloride resent a significant economic and welfare problem. -

A Single Amino Acid Substitution in the Third Transmembrane Region Has Opposite Impacts on the Selectivity of the Parasiticides
Supplemental material to this article can be found at: http://molpharm.aspetjournals.org/content/suppl/2017/09/08/mol.117.109413.DC1 1521-0111/92/5/546–555$25.00 https://doi.org/10.1124/mol.117.109413 MOLECULAR PHARMACOLOGY Mol Pharmacol 92:546–555, November 2017 Copyright ª 2017 by The American Society for Pharmacology and Experimental Therapeutics A Single Amino Acid Substitution in the Third Transmembrane Region Has Opposite Impacts on the Selectivity of the Parasiticides Fluralaner and Ivermectin for Ligand-Gated Chloride Channels s Yunosuke Nakata, Toshinori Fuse, Kohei Yamato, Miho Asahi, Kunimitsu Nakahira, Fumiyo Ozoe, and Yoshihisa Ozoe Faculty of Life and Environmental Science, Shimane University, Matsue, Shimane, Japan (Y.N., T.F., K.Y, F.O., Y.O.); and Downloaded from Biological Research Laboratories, Nissan Chemical Industries, Ltd., Saitama, Japan (M.A., K.N.) Received May 16, 2017; accepted September 9, 2017 ABSTRACT Fluralaner (Bravecto) is a recently marketed isoxazoline ectopar- transmembrane region (TM3) with an aromatic amino acid molpharm.aspetjournals.org asiticide. This compound potently inhibits GABA-gated chloride dramatically enhanced the potency of fluralaner in the GluCls. channels (GABACls) and less potently glutamate-gated chloride In stark contrast to the enhancement of fluralaner potency, this channels (GluCls) in insects. The mechanism underlying this mutation eliminated the activation of currents and the potenti- selectivity is unknown. Therefore, we sought to identify the ation but not the antagonism of glutamate responses that are amino acid residues causing the low potency of fluralaner toward otherwise all elicited by the macrolide parasiticide ivermectin GluCls. We examined the fluralaner sensitivity of mutant housefly (IVM). -

Study Protocol
STUDY PROTOCOL FOR COMPASSIONATE AQUACULTURE INVESTIGATIONAL NEW ANIMAL DRUG (INAD) EXEMPTION FOR 35% PEROX-AID® (HYDROGEN PEROXIDE) FOR THE CONTROL OF ECTOPARASITES (INAD #11-669) Sponsor: U.S. Fish and Wildlife Service, Division of the National Fish Hatchery System ______________________ ___________________ Sponsor Signature Date Approved Manufacturer: Eka Chemicals Inc. 1775 West Oak Commons Court Marietta, Georgia 30062-2254 Facility for Coordination of 35% PEROX AID® INAD: Aquatic Animal Drug Approval Partnership Program 4050 Bridger Canyon Road Bozeman, Mt 59715 Proposed Starting Date: December 1, 2007 Proposed Ending Date: November 30, 2012 Study Director: Mr. Jim Bowker _________________________ ________________ Study Director Signature Date Clinical Field Trial Location and Trial Number: Facility ______________________________________________________________ Type or Print Name Investigator____________________________________________________________ Type or Print Name __________________________________________ ________________________ Investigator Signature Date 1 I. STUDY ID AND TITLE 3 II. SPONSOR 3 III. INVESTIGATORS/FACILITIES 4 IV. PROPOSED STARTING AND COMPLETION DATES: 4 V. BACKGROUND/PURPOSE 4 VI. SPECIFIC OBJECTIVES 6 VII. MATERIALS 7 VIII. EXPERIMENTAL UNIT 10 IX. ENTRANCE CRITERIA 10 X. TREATMENT GROUPS 11 XI. TREATMENT SCHEDULES 12 XII. TREATMENT RESPONSE PARAMETERS 15 XIII. FORMS FOR DATA COLLECTION 15 XIV. RECORD KEEPING PROCEDURES 16 XV. DISPOSITION OF INVESTIGATIONAL ANIMALS 16 XVI. DISPOSITION OF INVESTIGATIONAL -

Other Contributions
Other Contributions NATURE NOTES Amphibia: Anura Incilius luetkenii, Smilisca sordida, and Lithobates forreri. Predation by birds. Predation on adult anurans by tropical birds has been recorded on numerous occasions, where birds of various families (e.g., Accipitridae, Striigidae, Momotidae, Turdidae) have preyed on anurans of different families (e.g., Centrolenidae, Dendrobatidae, Hylidae, Leptodactylidae, Rhinophrynidae; Hayes, 1983; Master 1999; Toledo et al., 2007; Acosta and Morún, 2014; Ramírez-Fernández and Solís-DelValle, 2014). The majority of these events are opportunistic and associated with diet-generalist or invertebrate- and vertebrate-predator bird species (Toledo et al., 2007; Amézquita et al., 2013; Paluh et al., 2015). Here, we present information on the predation of Incilius luetkenii (Bufonidae), Smilisca sordida (Hylidae), and Lithobates forreri (Ranidae) by birds in Costa Rica. On 26 May 2013, at Área de Conservación Guanacaste, Sector Santa Rosa, Provincia de Guanacaste, Costa Rica (10°50'N, 85°37'W; WGS 84; elev. 298 m), we observed a Roadside Hawk (Buteo [Rupornis] magnirostris) feeding on an individual of Incilius luetkenii. The hawk was standing on the ground pecking and eating a dead I. luetkenii along the edge of gravel road in Tropical Dry Forest, but once it was startled flew across the road without the toad and perched on a tree approximately 5 m from the ground. We cannot determine if the hawk captured the toad or found it dead (the toad remains appeared fresh), but either of these behaviors was likely because the previous evening breeding aggregations involving I. luetkenii had occurred in the area. Although frogs and toads are import- ant dietary items for this hawk (Haverschmidt, 1972; Beltzer, 1990), this observation is the first to report I. -

Laboratory and Field Studies to Investigate the Efficacy of a Novel
Kryda et al. Parasites Vectors (2019) 12:445 https://doi.org/10.1186/s13071-019-3702-6 Parasites & Vectors RESEARCH Open Access Laboratory and feld studies to investigate the efcacy of a novel, orally administered combination product containing moxidectin, sarolaner and pyrantel for the prevention of heartworm disease (Diroflaria immitis) in dogs Kristina Kryda1*, Robert H. Six1, Kelly F. Walsh1, Susan J. Holzmer1, Sara Chapin1, Sean P. Mahabir1, Melanie Myers1, Tammy Inskeep1, Jady Rugg1, Blair Cundif1, Aleah Pullins1, Michael Ulrich2, John W. McCall3, Tom L. McTier1 and Steven J. Maeder1 Abstract Background: Diroflaria immitis is a flarial parasite of dogs that can cause serious or fatal cardiopulmonary disease. Three studies were conducted to evaluate the efcacy and safety of monthly treatment with moxidectin in a chew- able tablet product in combination with sarolaner and pyrantel to prevent heartworm disease in dogs after experi- mental challenge and in a clinical feld study in the USA. Methods: In two laboratory studies, dogs (8 per group) that had been inoculated 30 days prior with 50 third-stage D. immitis larvae were randomized to treatment on Day 0 with placebo or combination product, at the minimum dose of 24 µg/kg moxidectin, 2 mg/kg sarolaner and 5 mg/kg pyrantel (as pamoate salt). Study 2 also included groups treated with tablets containing moxidectin-alone (24 µg/kg) or sarolaner-alone (2 mg/kg). Efcacy was evaluated ~ 5 months after inoculation by adult heartworm counts at necropsy. In the feld study, 410 dogs 8 weeks-old from 23 USA veterinary clinics were treated for 11 months with either combination product at 24–48 µg/kg≥ moxidectin, 2–4 mg/kg sarolaner and 5–10 mg/kg pyrantel (n 272) or Heartgard® Plus (ivermectin/pyrantel) at the label recom- mended dose rate (n 138). -

Pharmaceuticals As Environmental Contaminants
PharmaceuticalsPharmaceuticals asas EnvironmentalEnvironmental Contaminants:Contaminants: anan OverviewOverview ofof thethe ScienceScience Christian G. Daughton, Ph.D. Chief, Environmental Chemistry Branch Environmental Sciences Division National Exposure Research Laboratory Office of Research and Development Environmental Protection Agency Las Vegas, Nevada 89119 [email protected] Office of Research and Development National Exposure Research Laboratory, Environmental Sciences Division, Las Vegas, Nevada Why and how do drugs contaminate the environment? What might it all mean? How do we prevent it? Office of Research and Development National Exposure Research Laboratory, Environmental Sciences Division, Las Vegas, Nevada This talk presents only a cursory overview of some of the many science issues surrounding the topic of pharmaceuticals as environmental contaminants Office of Research and Development National Exposure Research Laboratory, Environmental Sciences Division, Las Vegas, Nevada A Clarification We sometimes loosely (but incorrectly) refer to drugs, medicines, medications, or pharmaceuticals as being the substances that contaminant the environment. The actual environmental contaminants, however, are the active pharmaceutical ingredients – APIs. These terms are all often used interchangeably Office of Research and Development National Exposure Research Laboratory, Environmental Sciences Division, Las Vegas, Nevada Office of Research and Development Available: http://www.epa.gov/nerlesd1/chemistry/pharma/image/drawing.pdfNational -

Armand Bayou Watershed Plan Cover: Top Left Photo Courtesy Armand Bayou Nature Center; All Other Photos © Cliff Meinhardt Armand Bayou Watershed Plan Phase I
Armand Bayou Watershed Plan COVER: TOP LEFT PHOTO COURTESY ARMAND BAYOU NATURE CENTER; ALL OTHER PHOTOS © CLIFF MEINHARDT Armand Bayou Watershed Plan Phase I A Report of the Coastal Coordination Council Pursuant to National Oceanic and Atmospheric Administration Award No. NA170Z1140 Production of this document supported in part by Institutional Grant NA16RG1078 to Texas A&M University from the National Sea Grant Office, National Oceanic and Atmospheric Administration, U.S. Department of Commerce, and a grant from ExxonMobil Coporation ii PHOTO © CLIFF MEINHARDT Contents Acknowledgements .......................................................................................................................................................1 Executive Summary .......................................................................................................................................................2 Introduction .............................................................................................................................................................2 The Armand Bayou Watershed Partnership ..................................................................................................................2 State of the Watershed ..............................................................................................................................................2 Institutional Framework ..............................................................................................................................................3 -

BULLETIN Chicago Herpetological Society
BULLETIN of the Chicago Herpetological Society Volume 53, Number 8 August 2018 BULLETIN OF THE CHICAGO HERPETOLOGICAL SOCIETY Volume 53, Number 8 August 2018 Notes on the Herpetofauna of Western Mexico 19: An Update to the Herpetofauna of Volcán de Tequila in Jalisco, Mexico . .Juan Rubén Rojo-Gutiérrez, David Jaimes-Rodríguez, Daniel Cruz-Sáenz, Laura López-Fernández, Enrique Chávez-Uribe and David Lazcano 165 Toad Stools: Part Two . Dennis A. Meritt Jr. 170 The Digital Age and Herp Lit: An Evolving Playing Field in the New Millennium . R. Michael Burger 171 Deputy Louie . Roger A. Repp 174 What You Missed at the June Meeting: Daniel Parker . .John Archer 178 Herpetology 2018......................................................... 181 Advertisements . 184 New CHS Members This Month . 184 Cover: Coral-bellied ring-necked snake, Diadophis punctatus pulchellus, Tuolumne County, California. Photograph by Stephen L. Barten, DVM. STAFF Membership in the CHS includes a subscription to the monthly Bulletin. Annual dues are: Individual Membership, $25.00; Editor: Michael A. Dloogatch --- [email protected] Family Membership, $28.00; Sustaining Membership, $50.00; Copy editor: Joan Moore Contributing Membership, $100.00; Institutional Membership, $38.00. Remittance must be made in U.S. funds. Subscribers 2017 CHS Board of Directors outside the U.S. must add $12.00 for postage. Send membership dues or address changes to: Chicago Herpetological Society, President: Rich Crowley Membership Secretary, 2430 N. Cannon Drive, Chicago, IL 60614. Vice-president: Jessica Wadleigh Treasurer: John Archer Manuscripts published in the Bulletin of the Chicago Herpeto- Recording Secretary: Gail Oomens logical Society are not peer reviewed. Manuscripts and letters Media Secretary: Kim Klisiak concerning editorial business should be e-mailed to the editor, Membership Secretary: Mike Dloogatch [email protected].