Guideline on the Summary of Product Characteristics for Antiparasitic Veterinary Medicinal Products EMA/CVMP/EWP/170208/2005-Rev.1 Page 2/14
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Skin Probiotics ACCELERATING INNOVATION
ACCELERATING INNOVATION James Madison University technologies are available for licensing through its nonprofit affiliate, James Madison Innovations, Inc. Skin Probiotics Inventors: Reid Harris and Kevin Minbiole Department: Biology and Chemistry Overview Research Funding and Source: National Science Foundation James Madison University inventors have filed a patent Years of Development: 4 years application on a helpful bacterium that could potentially be Technology Readiness: Research Development used as a therapy for human skin fungus such as athlete’s foot. Patent Status: U.S. patent pending 20110002891 The JMU inventors have developed a potential process to Contact: Mary Lou Bourne, Director of Technology Transfer deliver a helpful microbe, Janthinobacterium Lividum to the James Madison University skin using a pharmaceutically acceptable carrier. The microbe Phone: (540)568-2865 E-mail: [email protected] has been shown to suppress bacterial and fungal growth on animals and in lab demonstrations. Tech Transfer and Business Model Infections can be a problem for a wide array of hosts. For JMI is interested in identifying an existing company or example, there are a variety of infections, such as bacterial, entrepreneur interested in commercializing the technology viral and/or fungal infections, that affect a large percentage of either under an exclusive or a non-exclusive license. A the human population. Tricophyton rubrum, the fungus that small company or entrepreneur could further develop the causes athlete’s foot, is responsible for approximately 46% to technology, possibly using SBIR or STTR funding. 72% of cutaneous and nail mycoses worldwide. Onychomycosis, a common and persistent fungal infection, is Market and Competition In 2008, the U.S. -

Lindane Lotion USP, 1% RX Only WARNINGS
Lindane Lotion USP, 1% RX Only WARNINGS: Lindane Lotion should only be used in patients who cannot tolerate or have failed first-line treatment with safer medications for the treatment of scabies. (See INDICATIONS AND USAGE.) Neurologic Toxicity Seizures and deaths have been reported following Lindane Lotion use with repeat or prolonged application, but also in rare cases following a single application used according to directions. Lindane lotion should be used with caution for infants, children, the elderly, and individuals with other skin conditions (e.g, atopic dermatitis, psoriasis) and in those who weigh < 110 lbs (50 kg) as they may be at risk of serious neurotoxicity. Contraindications Lindane Lotion is contraindicated in premature infants and individuals with known uncontrolled seizure disorders. Proper Use Instruct patients on the proper use of Lindane Lotion, the amount to apply, how long to leave it on, and avoiding re-treatment. Inform patients that itching occurs after the successful killing of scabies and is not necessarily an indication for re-treatment with Lindane Lotion. (See DOSAGE AND ADMINISTRATION.) DESCRIPTION Lindane Lotion USP, 1%, is an ectoparasiticide and ovicide. In addition to the active ingredient, lindane, it contains glycerol monostearate, cetyl alcohol, stearic acid, trolamine, carrageenan, 2- amino-2-methyl-1-propanol, methylparaben, butylparaben, perfume and water to form a lotion. Lindane is the gamma isomer of 1,2,3,4,5,6-hexachlorocyclohexane having the following structural formula: C6H6Cl6 M.W. 290.83 CLINICAL PHARMACOLOGY Lindane Lotion USP, 1%, is an ectoparasiticide and ovicide effective against Sarcoptes scabiei (scabies). Lindane exerts its parasiticidal action by being directly absorbed into the parasites and 1 their ova. -

Revised Use-Function Classification (2007)
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY IPCS INTOX Data Management System (INTOX DMS) Revised Use-Function Classification (2007) The Use-Function Classification is used in two places in the INTOX Data Management System: the Communication Record and the Agent/Product Record. The two records are linked: if there is an agent record for a Centre Agent that is the subject of a call, the appropriate Intended Use-Function can be selected automatically in the Communication Record. The Use-Function Classification is used when generating reports, both standard and customized, and for searching the case and agent databases. In particular, INTOX standard reports use the top level headings of the Intended Use-Functions that were selected for Centre Agents in the Communication Record (e.g. if an agent was classified as an Analgesic for Human Use in the Communication Record, it would be logged as a Pharmaceutical for Human Use in the report). The Use-Function classification is very important for ensuring harmonized data collection. In version 4.4 of the software, 5 new additions were made to the top levels of the classification provided with the system for the classification of organisms (items XIV to XVIII). This is a 'convenience' classification to facilitate searching of the Communications database. A taxonomic classification for organisms is provided within the INTOX DMS Agent Explorer. In May/June 2006 INTOX users were surveyed to find out whether they had made any changes to the Use-Function Classification. These changes were then discussed at the 4th and 5th Meetings of INTOX Users. Version 4.5 of the INTOX DMS includes the revised pesticides classification (shown in full below). -

Natural Products As Potential Antiparasitic Drugs
Natural Products as potential antiparasitic drugs OLIVER KAYSER1, ALBRECHT F. KIDERLEN2, SIMON L. CROFT3 1Freie Universität Berlin Institut für Pharmazie, Pharmazeutische Biotechnologie Kelchstraße 31, 12169 Berlin, Germany 2Robert Koch-Institut Nordufer 20 13353 Berlin, Germany 3London School of Hygiene and Tropical Medicine Department of Infectious and Tropical Diseases Keppel Street London, WC1E 7HT, United Kingdom ABSTRACT: Pharmaceutical research in natural products represents a major strategy for discovering and developing new drugs. The use of medicinal plants for the treatment of parasitic diseases is well known and documented since ancient times e.g. by the use of Cinchona succiruba (Rubiaceae) as an antimalarial. This chapter provides a comprehensive review of the latest results in the field of antiparasitic drug development from biologic sources (plants, bacteria, fungi and marine organisms) focussing on the treatment of protozoal infections (Plasmodium, Leishmania, Trypanosoma spp.). The status of validated in vitro and in vivo assays is reviewed, discussing their different features, problems and limitations. Because of the high number of natural products tested against the aforesaid protozoa in the last years, we limit the discussion to lignans, phenolics, terpenoids, and alkaloids as defined natural product classes. The review also covers essential research topics of recent publications on specific natural products (e.g. licochalcone A, benzyl- and naphthylisoquinoline alkaloids, and artemisinin) and gives an outlook to semi- synthetic approaches of drugs already introduced in clinics or in clinical trial studies. 1. INTRODUCTION The fascination of natural products, mostly as used as a preparation from a plant with known medicinal properties, goes back to ancient times. The discovery of pure compounds as active principles in plants was first described at the beginning of the 19th century, and the art of exploiting natural products has become part of the molecular sciences. -

Antibiotics May Eradicate Gastrointestinal Immunodeficiency.8
1122 Letters to the Editor J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.54.12.1122 on 1 December 1991. Downloaded from sitivity.? We present a case of OMM without pseudobulbar palsy. The association of vitro.8 Whipple's disease is associated with symptoms of Whipple's disease and with supranuclear gaze paresis with oculomas- immunodeficiency.8 Thus intestinal wall negative peroral jejunoileal biopsies, which ticatory myorhythmia, however, seems to be macrophages are ineffective in phagocytosing indicates the usefulness of laparotomy for pathognomonic of the effects of Whipple's intracellular gram positive bacilli, resulting in jejunoileal biopsies as an alternative to brain disease on the CNS.'2 This led us to perform inability to eliminate chronic infection.9 This biopsy to confirm Whipple's disease. surgical jejunal and mesenteric lymph node suggests that Whipple's disease may be con- A 47 year old woman was admitted in May biopsies rather than a brain biopsy despite sidered as a disease of macrophages.8 The 1988 in a depressed state. In June 1987 she negative endoscopic and numerous peroral periventricular and periaqueductal distribu- had noted progressive visual disturbance. distal jejunal and ileal biopsies. The nor- tion of the CNS involvement in Whipple's Rhythmic elevations of her right upper lip mality ofthe CT and MRI scans also suppor- disease consists of macrophagic infiltration appeared, and later, paroxysmal hypersomnia ted this decision. and subependymal nodules. Such a and considerable weight gain (10 kg). A nine The possibility of brain involvement with- "tumoural" involvement may explain why month course oftreatment for depression was out systemic manifestation in Whipple's dis- antibiotics with good BBB diffusion are not ineffective and her symptoms and signs ease should be kept in mind. -

Antiparasitic Properties of Cardiovascular Agents Against Human Intravascular Parasite Schistosoma Mansoni
pharmaceuticals Article Antiparasitic Properties of Cardiovascular Agents against Human Intravascular Parasite Schistosoma mansoni Raquel Porto 1, Ana C. Mengarda 1, Rayssa A. Cajas 1, Maria C. Salvadori 2 , Fernanda S. Teixeira 2 , Daniel D. R. Arcanjo 3 , Abolghasem Siyadatpanah 4, Maria de Lourdes Pereira 5 , Polrat Wilairatana 6,* and Josué de Moraes 1,* 1 Research Center for Neglected Diseases, Guarulhos University, Praça Tereza Cristina 229, São Paulo 07023-070, SP, Brazil; [email protected] (R.P.); [email protected] (A.C.M.); [email protected] (R.A.C.) 2 Institute of Physics, University of São Paulo, São Paulo 05508-060, SP, Brazil; [email protected] (M.C.S.); [email protected] (F.S.T.) 3 Department of Biophysics and Physiology, Federal University of Piaui, Teresina 64049-550, PI, Brazil; [email protected] 4 Ferdows School of Paramedical and Health, Birjand University of Medical Sciences, Birjand 9717853577, Iran; [email protected] 5 CICECO-Aveiro Institute of Materials & Department of Medical Sciences, University of Aveiro, 3810-193 Aveiro, Portugal; [email protected] 6 Department of Clinical Tropical Medicine, Faculty of Tropical Medicine, Mahidol University, Bangkok 10400, Thailand * Correspondence: [email protected] (P.W.); [email protected] (J.d.M.) Citation: Porto, R.; Mengarda, A.C.; Abstract: The intravascular parasitic worm Schistosoma mansoni is a causative agent of schistosomiasis, Cajas, R.A.; Salvadori, M.C.; Teixeira, a disease of great global public health significance. Praziquantel is the only drug available to F.S.; Arcanjo, D.D.R.; Siyadatpanah, treat schistosomiasis and there is an urgent demand for new anthelmintic agents. -

WSAVA List of Essential Medicines for Cats and Dogs
The World Small Animal Veterinary Association (WSAVA) List of Essential Medicines for Cats and Dogs Version 1; January 20th, 2020 Members of the WSAVA Therapeutic Guidelines Group (TGG) Steagall PV, Pelligand L, Page SW, Bourgeois M, Weese S, Manigot G, Dublin D, Ferreira JP, Guardabassi L © 2020 WSAVA All Rights Reserved Contents Background ................................................................................................................................... 2 Definition ...................................................................................................................................... 2 Using the List of Essential Medicines ............................................................................................ 2 Criteria for selection of essential medicines ................................................................................. 3 Anaesthetic, analgesic, sedative and emergency drugs ............................................................... 4 Antimicrobial drugs ....................................................................................................................... 7 Antibacterial and antiprotozoal drugs ....................................................................................... 7 Systemic administration ........................................................................................................ 7 Topical administration ........................................................................................................... 9 Antifungal drugs ..................................................................................................................... -

Antiprotozoal and Antitumor Activity of Natural Polycyclic Endoperoxides: Origin, Structures and Biological Activity
molecules Review Antiprotozoal and Antitumor Activity of Natural Polycyclic Endoperoxides: Origin, Structures and Biological Activity Valery M. Dembitsky 1,2,*, Ekaterina Ermolenko 2, Nick Savidov 1, Tatyana A. Gloriozova 3 and Vladimir V. Poroikov 3 1 Centre for Applied Research, Innovation and Entrepreneurship, Lethbridge College, 3000 College Drive South, Lethbridge, AB T1K 1L6, Canada; [email protected] 2 A.V. Zhirmunsky National Scientific Center of Marine Biology, 17 Palchevsky Str., 690041 Vladivostok, Russia; [email protected] 3 Institute of Biomedical Chemistry, 10 Pogodinskaya Str., 119121 Moscow, Russia; [email protected] (T.A.G.); [email protected] (V.V.P.) * Correspondence: [email protected]; Tel.: +1-403-320-3202 (ext. 5463); Fax: +1-888-858-8517 Abstract: Polycyclic endoperoxides are rare natural metabolites found and isolated in plants, fungi, and marine invertebrates. The purpose of this review is a comparative analysis of the pharmacological potential of these natural products. According to PASS (Prediction of Activity Spectra for Substances) estimates, they are more likely to exhibit antiprotozoal and antitumor properties. Some of them are now widely used in clinical medicine. All polycyclic endoperoxides presented in this article demonstrate antiprotozoal activity and can be divided into three groups. The third group includes endoperoxides, which show weak antiprotozoal activity with a reliability of up to 70%, and this group includes only 1.1% of metabolites. The second group includes the largest number of endoperoxides, Citation: Dembitsky, V.M.; which are 65% and show average antiprotozoal activity with a confidence level of 70 to 90%. Lastly, Ermolenko, E.; Savidov, N.; the third group includes endoperoxides, which are 33.9% and show strong antiprotozoal activity with Gloriozova, T.A.; Poroikov, V.V. -
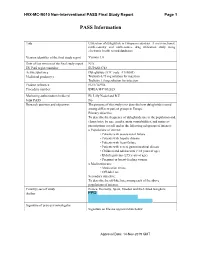
PASS Information
H9X-MC-B010 Non-interventional PASS Final Study Report Page 1 PASS Information Title Utilisation of dulaglutide in European countries: A cross-sectional, multi-country and multi-source drug utilisation study using electronic health record databases Version identifier of the final study report Version 1.0 Date of last version of the final study report N/A EU PAS register number EUPAS13783 Active substance Dulaglutide (ATC code: A10BJ05) Medicinal product(s): Trulicity 0.75 mg solution for injection Trulicity 1.5 mg solution for injection Product reference: EU/1/14/956 Procedure number: EMEA/H/C/002825 Marketing authorisation holder(s) Eli Lilly Nederland B.V. Joint PASS No Research question and objectives The purpose of this study is to describe how dulaglutide is used among different patient groups in Europe. Primary objective: To describe the frequency of dulaglutide use in the population and characterise by age, gender, main comorbidities, and main co- prescriptions overall and in the following subgroups of interest: o Populations of interest: • Patients with severe renal failure • Patients with hepatic disease • Patients with heart failure • Patients with severe gastrointestinal disease • Children and adolescents (<18 years of age) • Elderly patients (≥75 years of age) • Pregnant or breast-feeding women o Medication use: • Medication errors • Off-label use Secondary objective: To describe the off-label use among each of the above populations of interest. Country(-ies) of study France, Germany, Spain, Sweden and the United Kingdom Author PPD Signature of principal investigator Signature on file/see approval date below Approval Date: 14-Nov-2019 GMT H9X-MC-B010 Non-interventional PASS Final Study Report Page 2 Marketing Authorisation Holder Marketing authorisation holder (MAH) Eli Lilly Nederland B.V. -

A Saprolegnia Parasitica Challenge System for Rainbow Trout: Assessment of Pyceze As an Anti-Fungal Agent for Both Fish and Ova
DISEASES OF AQUATIC ORGANISMS Published May 12 Dis Aquat Org l A Saprolegnia parasitica challenge system for rainbow trout: assessment of Pyceze as an anti-fungal agent for both fish and ova T. G.Pottinger*, J. G. Day NERC Institute of Freshwater Ecology, Windermere Laboratory, Far Sawrey, Ambleside, Cumbria, LA22 OLP, United Kingdom ABSTRACT: A reproducible Saprolegnia parasitica spore delivery system was developed and demon- strated to be effective in providing a sustained spore challenge for up to 10 d. Treatment of rainbow trout with slow-release intraperitoneal implants containing cortisol resulted in chronically elevated blood cortisol levels and rendered the fish susceptible to infection by S. parasitica when exposed to the spore challenge. Sham-implanted fish were not susceptible to infect~on.Bronopol (2-bromo-2-nitro- propane-1,3-dol), formulated as Pyceze, was effective in protecting predisposed fish from infection by S. parasitica when administered as a daily bathlflush treatment at concentrations of 15 mg I-' and greater. Pyceze was also demonstrated to protect fertilised rainbow trout ova from S. parasitica chal- lenge when administered as a daily bath/flush treatment at concentrations of between 30 and 100 mg 1-l. Pyceze appears to qualify as a safe and effective replacement for malachite green and formalin in the prevention of fungal infections in the aquaculture environment. KEY WORDS: Saprolegnia . Fungal infection . Salmonid . Bronopol - Pyceze . Cortisol INTRODUCTION chite green in combating mycotic infections of fish and fish eggs, but is safer for the operator, the fish, and Mycotic infections of farmed fish, primarily by water the environment. Among the alternative compounds moulds or pseudofungi of the genus Saprolegnia, rep- tested for antifungal activity are sodium chloride resent a significant economic and welfare problem. -

The Role of Antiparasitc Drugs and Steroids in Covid-19 Treatment
Research, Society and Development, v. 10, n. 8, e39510817300, 2021 (CC BY 4.0) | ISSN 2525-3409 | DOI: http://dx.doi.org/10.33448/rsd-v10i8.17300 The role of antiparasitc drugs and steroids in Covid-19 treatment O papel das drogas antiparasitárias e corticóides no tratamento da Covid-19 El papel de los antiparasitos y los esteroides en el tratamiento del Covid-19 Received: 06/17/2021 | Reviewed: 06/25/2021 | Accept: 07/03/2021 | Published: 07/14/2021 Luciano Barreto Filho ORCID: https://orcid.org/0000-0002-1508-4812 Faculdade de Odontologida do Recife, Brazil E-mail: [email protected] Paulo Reis Melo Júnior ORCID: https://orcid.org/0000-0001-9926-5348 Faculdade de Odontologia do Recife, Brazil E-mail: [email protected] Guilherme Marinho Sampaio ORCID: https://orcid.org/0000-0003-4441-7601 Faculdade de Odontologia do Recife, Brazil E-mail: [email protected] Gabriel Henrique Queiroz Oliveira ORCID:https://orcid.org/0000-0002-7795-3964 Faculdade de Odontologia do Recife, Brazil E-mail: [email protected] Hadassa Fonsêca Da Silva ORCID: https://orcid.org/0000-0002-9432-9522 Faculdade de Odontologia do Recife, Brazil E-mail: [email protected] Sandra Sayão Maia ORCID: https://orcid.org/0000-0001-6808-9775 Faculdade de Odontologia do Recife, Brazil E-mail: [email protected] Abstract Background: COVID-19 has emerged as a pandemic that spread throughout the world in less than 6 months, leaving hundred thousand deaths behind. Surprisingly, old drug arsenal has now been applied as an option of treatment. Objective: The aim of this article was to accomplish a literature review concerning the antiparasitic chloroquine, ivermectin, nitazoxanide; as well as glucocorticoids as possible therapeutic agents to be applied in patients with COVID-19 in Brazilian hospitals. -

Essential Oils and Bioactive Components Against Arthritis: a Novel Perspective on Their Therapeutic Potential
plants Review Essential Oils and Bioactive Components against Arthritis: A Novel Perspective on Their Therapeutic Potential Mariangela Marrelli * , Valentina Amodeo, Maria Rosaria Perri, Filomena Conforti y and Giancarlo Statti y Department of Pharmacy, Health and Nutritional Sciences, University of Calabria, 87036 Rende (CS), Italy; [email protected] (V.A.); [email protected] (M.R.P.); fi[email protected] (F.C.); [email protected] (G.S.) * Correspondence: [email protected]; Tel.: +39-0984-493168; Fax: +39-0984-493107 These authors jointly supervised and contributed equally to this work. y Received: 18 August 2020; Accepted: 21 September 2020; Published: 23 September 2020 Abstract: Essential oils (EOs) are known to possess a number of beneficial properties. Their antimicrobial, anti-inflammatory, antioxidant, antidiabetic, and cancer-preventing activities have been extensively reported. Due to their wide use as food preservers and additives, as well as their use in agriculture, perfumes, and make-up products, these complex mixtures of volatile compounds have gained importance from a commercial point of view, not only in the pharmaceutical industry, but also in agronomic, food, cosmetic, and perfume industries. An analysis of the recent scientific literature allowed us to highlight the presence of an increasing number of studies on the potential antiarthritic properties of EOs and their main constituents, which seems to suggest a new interesting potential therapeutic application. The aim of this review is to examine the current knowledge on the beneficial effects of essential oils in the treatment of arthritic diseases, providing an overview of the reports on the in vivo and in vitro effects of EOs.