The Impacts of Honey Bee Queen Stress on Worker Behavior and Health
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Honey Farming by ROB Manley
HONEY FARMING by R. O. B. MANLEY FABER AND FABER LTD 24 Russell Square London First published in mcmxlvi by Faber and Faber Limited 24 Russell Square London W.C. 1 Second Impression September mcmxlvi Third impression September mcmxlviii Printed in Great Britain by Latimer Trend & Co Ltd Plymouth All rights reserved PREFACE he writing of this little book about bee-farming and honey Tproduction was suggested by the many letters I have received during the past ten years, since the book Honey Production in the British Isles was published. I have tried to make it as useful and interesting as I can, but I am very well aware of its imperfections. To write an interesting account of technical operations and methods of working is not always easy. Honey Farming is not written for the novice, and in writing it I have assumed some considerable experience on the part of the reader; but I am now working on another book intended expressly for the beginner who wishes to take up the business of beekeeping as distinct from making a hobby of it, a rather difficult thing to do. I have to thank those friends who have assisted me with the photographs used in this book, especially Mr. C. P. Abbott who did most of them. The drawings were made by Mr. R. W. Ford of Reading. R. O. B. MANLEY CONTENTS Preface page 5 I. Retrospect 11 II. Essentials 28 III. Climate, Pasturage, and Apiaries 42 IV. Apiary Equipment 57 V. Breeding Bees 83 VI. The Passing Seasons 124 VII. -

AABA Newsletter
The Arundel Anne Arundel Beekeepers Association Newsletter Issue No. 23 January, 2016 was going on in my hives just a few minutes after I closed them up. Other The President Speaks AABA members at meetings would ask me about my hives, and I would answer By Lindsay Barranco vaguely that they were “fine” but honestly, it took me some time to get on Hello and Happy New Year! I top of what was happening in each hive. hope everyone had a wonderful holiday In time, I began keeping notes after and I wish each of you a healthy and looking in each hive, so I could at least happy new year. We had a busy and remember what I saw and could give interesting year in 2015. The AABA thought to what steps to take next. I participated in the University of probably held off on taking notes Maryland Bee Lab’s Sentinel Hive because I thought it would turn Research Project, which was fascinating, beekeeping into a real chore. To the rewarding and accumulated helpful data contrary, it helped me remember and to from around Maryland (and beyond) and make better management decisions. So, gave us specific information about hive if you resolve to do one thing differently weight, varroa and nosema levels in the this coming year, give it a try this Arlington Echo beehives. The final coming season and know what is report and short description of the happening in your hives – keep some research project are included in this notes and keep up with the needs of your newsletter. -

BEEKEEPING: General Information by R
BEEKEEPING: General Information by R. A. Morse and E. J. Dyce A Cornell Cooperative Extension Publication Information Bulletin 90 The New York State College of Agriculture and Life Sciences is a statutory college of the State University, at Cornell University, Ithaca, N.Y. 2 BEEKEEPING: This bulletin provides general informa Honey Bee as a Pollinator tion about beekeeping that is not usually General Information included in current publications. Informa The pollination of agricultural crops is by R. A. Morse and E. J. Dyce tion on specific beekeeping problems can the most important contribution of honey be obtained by writing to the Office of bees to our national economy. Although Apiculture, Department of Entomology, the value of honey bees for pollination Contents Cornell University, Ithaca, NY 14853. cannot be estimated , it is many times the 2 Extent of Beekeeping Industry total value of both the honey and bees wax that they produce . Without cross 2 Honey Bee as a Pollinator Extent of Beekeeping Industry pollination many crops would not set seed 3 Who Keeps Bees? or produce fruit. Many insects other than In New York State about 8,500 people the honey bee can carry pollen from one 3 Where Bees Can Be Kept keep at least 125,000 colonies of honey plant to another; but in areas where agri 4 A Skilled Occupation bees. The annual production is about 8 culture has been intensified, such as the million pounds of honey and 120,000 fruit areas in New York State, the number 4 How to Acquire a Knowledge of pounds of beeswax. -

Beekeeper Volume I, II & III Ebook Beekeeping Naturally
The Practical Beekeeper Volume I, II & III EBook Beekeeping Naturally Copyright © 2004-2011 by Michael Bush All rights reserved. No part of this book may be reproduced or transmitted in any form or by any means without written permission of the author. Cover Photo © 2011 Alex Wild www.alexanderwild.com ISBN: 978-161476-084-9 X-Star Publishing Company Founded 1961 Dedication This book is dedicated to Ed and Dee Lusby who were the real pioneers of modern natural beekeeping methods that could succeed with the Varroa mites and all the other new issues. Thank you for sharing it with the rest of us. About the Book This book is about how to keep bees in a natural and practical system where they do not require treatments for pests and diseases and only minimal interventions. It is also about simple practical beekeeping. It is about reducing your work. It is not a main-stream beekeeping book. Many of the concepts are contrary to “conventional” beekeeping. The techniques presented here are streamlined through decades of experimentation, adjustments and simplification. The content was written and then refined from responding to questions on bee forums over the years so it is tailored to the questions that beekeepers, new and experienced, have. It is divided into three volumes and this edition contains all three: Beginning, Intermediate and Advanced. Acknowledgments I’m sure I will forget to list many who have helped me along this path. For one thing many were often only known by the names they used on the many bee forums where they shared their experiences. -

South Africa
SOUTH AFRICA LOCAL SUPPLIER OF QUALITY BEEKEEPING EQUIPMENT Equipment Price BASIC HIVE – WAKSOL DIPPED Waksol Dipped Beehive (Brood Chamber only) R900.00 (incl. Floorboard, Entrance, Brood Chamber, 10 x Brood Frames with wax strips, Inner Cover, Telescopic Roof + Aluminum Sheet) Waksol Dipped Super + 10 Frames w/o wax strips R330.00 Queen Excluder – Metal R110.00 Queen Excluder – Plastic R60.00 Propolis Paste – a bee attractant / smear interior of fresh hive R100.00 Bee Hive Stand R480.00 BEESWAX Brood Sheet Foundation (each) R25.00 Beeswax – Cupcake (±100g) R40.00 TRAP HIVES (depends on availability) 6 Frame Trap Hive (Treated Wood with Entrance & Roof with R390.00 Aluminum Sheet, with 6 Frames – with wax foundation strips) October 2018 ACCESSORIES 9 Frame Spacers (pair) R13.00 Hive Tool – Wooden Handle R75.00 Hive Tool – Stainless Steel R100.00 Hive Tool – “Jero” (European Imported) Stainless Steel R140.00 Hive Tool – “J” Type R90.00 Hive Tool - Italian R60.00 Hive Tool – Crestadeira/Portuguese Long Tube Rod R140.00 Honey Scraper R40.00 Frame Grip R95.00 Plastic Honey Gate with Nut (white/orange) R140.00 Stainless Steel Honey Gate R 250.00 Stainless Steel Smoker – Large R600.00 Stainless Steel Smoker – Medium R500.00 Smoker Inlet – Large R30.00 Air Bellow + Wooden Back R70.00 Black Double Brood Frame Feeder R120.00 Plastic Feeders – Bottle Top R10.00 Plastic Feeders – Booster Bee R15.00 Plastic Entrance Feeder Cup R 30.00 Hive Strapping with Buckle (3 meters) R15.00 Bee Brush – Bristle Brush R70.00 Frame Wire – Stainless Steel (500g) -

November 2017 WARWICKSHIRE BEEKEEPER November 2017 Issue No: 678
Warwickshire Beekeeper November 2017 WARWICKSHIRE BEEKEEPER November 2017 Issue No: 678 Warwickshire County Honey Show The theme of the Branch Composite Display this year was ‘The Hobbit’ to mark the 80th Anniversary of the publication of JRR Tolkein’s book. This is the entry by Solihull Branch Photo: Valli Cawte Newsletter of the Warwickshire Beekeepers’ Association Registered Charity No. 500276 Warwickshire Beekeeper November 2017 Keep up to date with the latest research CENTRAL ASSOCIATION OF BEEKEEPERS Woodside Hotel, Kenilworth 17th – 19th November www.cabk.org.uk Warwickshire Events 7 Nov Branch Meeting Nuneaton & Being a Professional Beekeeper David McDowell Atherstone Hatters Space Community Centre, Upper Abbey Street, Nuneaton, CV11 5DN 13 Nov 7.30 Second Monday Meeting Shipston The Gate Inn, Upper Brailes, OX15 5AX 13 Nov 7.30 Branch Meeting Solihull Bees of Great Britain and Ireland Steven Falk Methodist Hall, Blossomfield Road, Solihull, B91 1LG 15 Nov 7.30 Branch Meeting Rugby The Stewarton Hive Will Messenger Friends Meeting House, Regent Street, Rugby 16 Nov Chairman’s Evening Talk on the RNLI Sutton Erdington Methodist Centre Coldfield 20 Nov 7.30 Branch Meeting Coventry Honey Bee Democracy Justus Klaar All Saints Church Hall, Rectory Lane, Allesley, CV5 9EQ 22 Nov 7.30 North Cotswold Lecture The World of the Honeybee Queen Celia Davis Adlestrop Village Hall, GL56 0UN 24 Nov 7.30 Branch Meeting Speaker tbc Birmingham Dame Elizabeth Cadbury Hall, Firbank Close, Bournville, B30 1UA 2 Warwickshire Beekeeper November 2017 6 Dec 7.30 Branch Meeting Rugby Christmas Quiz - Details to be announced Friends Meeting House, Regent Street, Rugby 9 Dec Noon Christmas Lunch Birmingham for Westley Hotel, Westley Road, Acocks Green. -

Queen Rearing Simplified
m s l:/ I, J !l ^.-, x^ \ "mmmmwm- Qlifp i. 1. Hill iGtbrara 5Jiirth Qlarolina ^tatp (ToUpap SF525 S55 ^ S00314368 P >n V This book may be kept out TWO WEEKi ONLY, and is subject to a fine of FIVE CENTS a day thereafter. It is due on the day indicated below: JUL 2 3 980 230- Po./ SEP 311^ jAN 4 1984 M^ 2 a 1954 50M—May-54—Form Digitized by the Internet Archive in 2009 with funding from NCSU Libraries http://www.archive.org/details/queenrearingsimpOOsmit Tilts book is dedicated to my full pm'tner — my wife. QUEEN REARING SIMPLIFIED BY JAY SMITH ytt^^^T- Vuhluhed hy THE A. 1. ROO'I" COMPANY MEDINA. OHIO 1923 CopyriKlit. I !»!!:!. liy 'I'he A. I. Root ('(iin|ian.v Made ill II. S. A. CHAPTERS. riiiijitcr T. • liii|p()rt,uice of (jotxl Queens. Chapter II. —Conditions Under Wliicli tlie Bees Re;ir Queens. Chapter III. —Queen Rearing for the Small Beekeeper. Cliapter IV. —Rejiring Queens on a Large Scale. Chapter V. — Dipping Cells. Chapter VI. —Royal Jelly. Chapter VII. —The Swarm Bo.v. Chapter VIII. —Getting the Bees in Condition for Cell-Buildinj Chapter IX. —Cell-Finishing Colonies. Chapter X. —Filling the Swarm Box. Chapter XI. —Grafting the Cell Cups, Cliapter XII. —Emptying the Swarm Box. Cliapter XIIT. —The Pritchard Forced Cell-Starting Colony. Cliapter XIV. —Our Daily Program. Chapter XV. —Nucleus Hives. Cliapter XVI. —Virgin or Cell Introduction. Chapter XVI r. —Cell Introduction. Chapter XVIII. —Why Nuclei Tear Down Cells. Cliapter XIX. —Forming Nuclei. Chapter XX. —Misfortunes of the Queen-breeder. -

The Basics of Beekeeping
The Basics of Beekeeping by Members of Dunblane and Stirling Beekeepers' Association 2018 edition Contents 1 Introduction 6 1.1 Development of methods of beekeeping . 6 1.2 The beekeeper . 8 1.2.1 Stings . 9 1.3 The place of the honey bee in nature . 11 1.4 Significant bee forage plants in our Association area . 13 2 Beekeeping Equipment 14 2.1 Beehives and their accessories . 14 2.1.1 Introduction . 14 2.1.2 The National hive and its accessories . 15 2.2 Protective clothing . 18 2.3 Tools for working with bees . 18 2.4 Harvesting tools . 18 2.5 Miscellaneous and specialist items . 19 2.6 Appendix | other hive designs . 19 2.6.1 Introduction . 19 2.6.2 The (Unmodified) National hive and the Wormit Commercial hive . 19 2.6.3 The Smith hive . 20 2.6.4 The WBC hive . 20 2.6.5 Some more unusual hive designs . 20 3 Basic Honey Bee Biology 23 3.1 What is a honey bee? . 23 3.2 The anatomy of a honey bee . 23 3.2.1 The Head . 24 3.2.2 The Thorax . 24 3.2.3 The abdomen . 25 3.3 The Colony . 26 3.3.1 The queen bee . 27 3.3.2 Drones . 28 3.3.3 Workers . 29 3.4 Honey bee communication. 31 3.4.1 The importance of pheromones in honey bee communication . 31 3.4.2 Trophallaxis (Food sharing) . 31 3.4.3 Bee dances . 32 3 4 Handling: Spring and Summer Management 33 4.1 Beekeeping | basic handling skills . -

Downloads%2Fsb397901c&Usg=Aovvaw3bqvlzyo6ag Ltoexcrixz
Catch The Buzz™ ® www.BeeCulture.com BiosecurityBiosecurity For BeekeepersBeekeepers AppreciationAppreciation ForFor PollenPollen $4.99 BC_October_2020.indd 1 9/17/2020 6:31:04 PM BC_October_2020.indd 2 9/17/2020 6:31:04 PM Healthy Bees. Healthy Planet. Available through beekeeping supply stores. 866-483-2929 | nodglobal.com | [email protected] @NODAPIARY BC_October_2020.indd 3 9/17/2020 6:31:07 PM BC_October_2020.indd 4 9/17/2020 6:31:09 PM Bee Culture October Features . HIVE MONITORING CONFERENCE 25 Fourth International Conference Goes Virtual. DO BEEKEEPERS LIKE Jerry Bromenshenk SAVING MONEY? 67 Wintering indoors. WHAT MAKES ME HAPPIEST 30 John Miller Besides my husband, dog and bees. Jennifer Berry A CASE FOR PERMANENT INSULATION 68 AMERICAN HONEY PRODUCERS 35 Warmer in Winter, cooler in Summer. Legislative report Bruce Moechnig Eric Silva FLOWERING STRIPS = REGENERON SCIENCE TALENT 38 Meet Raina Jain. INFECTION 71 Raina Jain Mick Kuikowski TELLING THE BEES 46 Keeping them informed of changes. SWARM TEAM CAPTAIN 72 Elsie Czyzowska Stephen Bishop VARROA RESISTANCE 76 BEE VET 50 Is it really possible? Biosecurity for beekeepers. Dr. Tracy Farone Terry Combs OVERWINTERING NUCS 80 PRESCRIBED FIRE 53 David MacFawn It might be just what the bees need. Richard Hines AGAINST THE GRAIN 83 Appreciation for pollen. ZOOMING 56 Dr. Christine Bertz To a bee meeting. Tina Sebestyen WE’LL MEET AGAIN - ONLINE! 84 Charlotte Ekker Wiggins MINDING YOUR BEES AND CUES 58 Part 1: Interpreting fruit scents. EBOLA, COVID-19 & BEEKEEPERS 86 Becky Masterman & Bridget Mendel Richard Godfrey HAVE A BUZZ 61 UP CLOSE WITH A NORTHERN Leah Smith CALIFORNIA BEEKEEPER 89 Ettamarie Peterson 800.289.7668 Executive Publisher – Brad Root Associate Publisher, Senior Editor – Jerry Hayes, [email protected], Ext. -
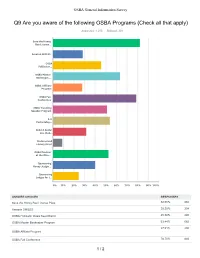
Survey Results
OSBA General Information Survey Q9 Are you aware of the following OSBA Programs (Check all that apply) Answered: 1,075 Skipped: 301 Save the Honey Bee License... Amazon SMILES OSBA Pollinator... OSBA Master Beekeeper... OSBA Affiliate Program OSBA Fall Conference OSBA Traveling Speaker Program 4-H Partnership... Build A Better Bee Club... Underserved Library Grant OSBA Pavilion at the Ohio... Sponsoring Honey Judgin... Sponsoring Judges for t... 0% 10% 20% 30% 40% 50% 60% 70% 80% 90% 100% ANSWER CHOICES RESPONSES Save the Honey Bee License Plate 82.05% 882 Amazon SMILES 28.28% 304 OSBA Pollinator Oasis Seed Blend 45.58% 490 OSBA Master Beekeeper Program 63.44% 682 27.91% 300 OSBA Affiliate Program OSBA Fall Conference 78.70% 846 1 / 2 OSBA General Information Survey OSBA Traveling Speaker Program 50.98% 548 4-H Partnership Program 53.67% 577 Build A Better Bee Club Workshop 31.53% 339 Underserved Library Grant 8.93% 96 OSBA Pavilion at the Ohio State Fair 52.56% 565 Sponsoring Honey Judging at the Ohio State Fair 40.00% 430 Sponsoring Judges for the 4-H Beekeeping senior level at the Ohio State Fair 24.56% 264 Total Respondents: 1,075 2 / 2 OSBA General Information Survey Q17 In 2018, were your bees inspected by a County Bee Inspector? Answered: 1,081 Skipped: 295 Yes No Do not have a county bee... 0% 10% 20% 30% 40% 50% 60% 70% 80% 90% 100% ANSWER CHOICES RESPONSES Yes 39.96% 432 No 60.04% 649 Do not have a county bee inspector 0.00% 0 TOTAL 1,081 1 / 1 OSBA General Information Survey Q15 Does your community have a beekeeping ordinance? Answered: 1,119 Skipped: 257 My community does not all.. -

Beekeeping Letters to the Nancy Ostiguy
For the queen bee!!! Beeswax Honey Comb Sheets in assorted colors to create your own candles!! 15 beautiful colors to choose from! Carton of 1 0 sheets with wicking and 5 lbs. of assorted honey candy $ 2 0 . 0 0 ! ! ! It’s a sweetheart of a deal! - Careon of 5 CM hive with 5^ ftames & 5^ sheetA of plasUcelL. Sweeeheart Special - $99.50 # 2 - Carton of 5 CM 6 518" Supers with 50 frames & 50 sheets of Plasttcell^. Sweetheart Special - $80.00 # 3 - Carton of 5 CM 5 ttlt6 '' Supers with 50 frames &50 sheets of Piasticel^. Sweetheart Special - $75.00 # 4 - Plas^ Helmet & Folding Veit and Leather Gloves - $28.00! # 5 - Budget ^^!versib(e Cedar Bottomd - $3.80 each/f W ESTEEINBB Serving the commercial beekeeper and the hobbyist for over 40 years. PO Box 190, Poison, MT 59860 Call Toll-Free 1-800-548-8440 Montana/Canadian Residents call 406-883-2918 or fax us at 406-883-4336. www.westernbee.com - order online!! We ship UPS, USPS and by common carrier. Orders over 200# can be shipped at reduced rates. February specials good through 3/10/2010. 'W Volume 150 No. 2 February 2010 Email [email protected] Web ontents www.americanbeejournal.com Editor-Joe M. Graham Advertising Manager-Marta Menn Samuel Wagner: Publishing Department- Dianne Behnke & Amy Hill First ABJ Editor — 127 • Samuel Wagner - The First Editor of the American Bee Journal Kent L. Pellett................................................................................................127 • The Home of the Honey Bee Has Changed Over Time G.H. Cale....................................................................................................... -

The Boxgrove Bugle
THE BOXGROVE BUGLE THE MAGAZINE LINKING CHURCH AND PARISH Boxgrove Priory, The Priory Vicarage, Church Lane, Boxgrove, Chichester, PO18 0ED Phone: +44 (0)1243 774045 ~ Fax: +44 (0)1243 774045 ~ www.boxgrovepriory.co.uk November 2019 Members of the congregation enjoying the Harvest Lunch on 6 October INSIDE THIS MONTH’S ISSUE Who's Who in Boxgrove .......................................... 2 'Match this!' ................................................................................. 13 From Fr Ian ................................................................................... 3 Boxgrove School Harvest Festival ................... 15 Other useful contact details ................................ 3 Boxgrove WI Centenary - report .............. 16 About The Bugle ..................................................................... 4 'Love me as you are' - a prayer ...................... 16 Priory Sunday School ..................................................... 5 Recipe: Fancy Mash ........................................................ 18 Boxgrove & Tangmere Julian Group .......... 5 Parish Pilgrimage to Walsingham ................. 19 Parish Calendar & Intentions ................................ 6 Cathedral lunchtime concerts .............................. 20 Boxgrove Parish Council report ........................ 7 WSCC Mobile Library consultation ....... 20 Companion Dog Show - report ..................... 8 School eligibility, September 2020 ............. 21 Forthcoming Events in the Priory ............... 9 Can you help the