In the Case of Robert Andrews Millikan
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Millikan's Struggle with Theory
FEATURES Did the great experimentalist doctor his own findings to suit his beliefs? Perhaps not, as aclose look at his beliefs reveals. Millikan's struggle with theory Gerald Holton n 1916 Millikan published his experi by any other experimenter. rectly at the ordinates, he decided"simply I mental determination of the value of Several factors were responsible for the to cut the feet off"where those curves were Planck's constant, with remarkable preci success of the experiment, performed at trailing on too far. sion. Itwas later cited as part ofhis Nobel the Ryerson Laboratory ofthe University In short, a triumphant work by a su Prize award. But as a study of that paper ofChicago. First ofall, Millikan's use ofan perbly confident scientist, ofhighest im together with Millikan's subsequent com ingenious device he termed "a machine portance in its day, and richly deserving to ments over the years reveal, he initiallyre shop in vacuo;' assembled thanks to "the be cited as part of his Nobel Prize award fused to accept the interpretation of the skill and experience of the mechanician, in 1923, "for his work on the elementary theoretical meaning ofhis work, as seen Mr. Julius Pearson" .Essentially, the device charge ofelectricityandthe photoelectric from the perspective of a present-day allowed a rotating sharp knife, controlled effect". If historically minded scholars physicist.Yet, over time, Millikan adjusted from outside the evacuated glass contain look through that 1916 volume of the his view retroactively to accept the mean er by electromagnetic means, to clean off Physical Review inwhich Millikan's paper ing ofwhat he had done - namely to pro the surface of the metal used (sodium, appeared, they will notice that physics in vide crucial support for Einstein's heuris potassium, lithium) before exposing it to America at that time was still a mixed bag. -

Millikan Oil Drop Experiment
Experiment 4 1 Millikan Oil-Drop Experiment Physics 2150 Experiment 4 University of Colorado1 Introduction The fundamental unit of charge is the charge of an electron, which has the magnitude of 푒 = 1.60219 x 10-19 C. With the exception of quarks, all elementary particles observed in nature are found to have a charge equal to an integral multiple of the charge of an electron. (Quarks have charge 푒/3, but they are never observed as free particles). By convention, the symbol 푒 represents a positive charge, so the charge 푞 of an electron is 푞 = −푒. The first person to measure accurately the electron’s charge was the American physicist R. A. Millikan. Working at the University of Chicago in 1912, Millikan found that droplets of oil from a simple spray bottle usually carry a net charge of a few electrons. He built an apparatus in which tiny oil droplets fall between two horizontal capacitor plates while their motion is observed with a microscope. A voltage applied between the plates creates an electric field, which can exert an upward force on a charge droplet, counteracting the force of gravity. By studying the motion of a droplet in response to gravity, the electric field between the plates, and viscous drag due to the air, Millikan was able to calculate the charge on the droplet. He published data on 58 droplets and reported that the charge of an electron is 푒 = (1.592±0.003) x 10-19 C. Millikan’s value was a bit low (three 휎 below the accepted value) because he used a slightly inaccurate expression for the drag force due to air. -

Tournament-9 Round 12 Tossups 1
Tournament-9 Round 12 Tossups 1. This psychologist argued that "moving toward others," "moving against others," and "moving away from others" were the three unhealthy trends. She organized the Association for the Advancement of Psychoanalysis after being expelled from the New York Psychoanalytic Institute. She argued against the concept of the death instinct and libido in works such as Feminine Psychology and The Neurotic Personality of Our Time. (*) For 10 points, name this psychologist, who introduced the concept of womb envy. ANSWER: Karen Horney 030-09-6-12102 2. The magnitudes of constructs of this type are related to J-coupling constants in proton NMR spectroscopy, according to the Karplus equations. One construct of this type is also known as the "dihedral" type, and applies when four or more atoms are bonded in a straight chain. For a central molecule with two sigma bonds, the magnitude of a construct of this type (*) decreases by one-third when a lone pair is present, which is the major difference between a linear and a bent molecule. For 10 points, name these figures important in molecular geometry, which come in "bond" and "torsional" varieties, and are typically measured in degrees. ANSWER: angles [or torsional angles; or bond angles; or dihedral angles] 026-09-6-12103 3. This deity trips a figure named Lit and kicks him into a pyre. This deity, assisted by some items given by Grid, defeats Geirrod, but fails to wrestle Eli, who is really Old Age. This deity cannot pick up the cat of (*) Utgard-Loki, but does make Thialfi into his apprentice. -

Harvey Fletcher and Henry Eyring: Men of Faith and Science
Edward L. Kimball Harvey Fletcher and Henry Eyring: Men of Faith and Science The year 1981 saw the deaths of Harvey Fletcher and Henry Eyring, men of great religious faith whose superb professional achievements placed them in the first ranks of the nation's scientists. (See Steven H. Heath's "The Reconcilia- tion of Faith and Science: Henry Eyring's Achievement," this issue.) Both could be said to have had simple religious faith — not because they were un- complicated people incapable of subtlety, but because their religious character was early and firmly grounded in a few fundamentals. This freed them from a life of continuing doubt and struggle. The two men, seventeen years apart in age, had a kind of family relation- ship. Henry Eyring's uncle Carl Eyring (after whom BYU's Eyring Science Center is named) married Fern Chipman; Harvey Fletcher married her sister Lorena. After their spouses died, Harvey Fletcher and Fern Chipman Eyring married. As a result, Henry Eyring called him Uncle Harvey. But that was not unique. Nearly everyone else did, too. Harvey Fletcher was born in 1884 in a little frame house in Provo, Utah. Among his memories are attending the dedication of the Salt Lake Temple and shaking President Wilford Woodruff's hand. As a young boy, he recited a short poem at a program in the Provo Tabernacle; and after he finished, Karl G. Maeser, principal of the Brigham Young Academy, stopped him before he could resume his seat, put his hand on Harvey's head, and said, "I want this congregation to know that this little boy will one day be a great man." Instead of being pleased, Harvey was bothered; he perceived it as a prediction of politi- cal leadership, which he did not want. -

Moremore Millikan’S Oil-Drop Experiment
MoreMore Millikan’s Oil-Drop Experiment Millikan’s measurement of the charge on the electron is one of the few truly crucial experiments in physics and, at the same time, one whose simple directness serves as a standard against which to compare others. Figure 3-4 shows a sketch of Millikan’s apparatus. With no electric field, the downward force on an oil drop is mg and the upward force is bv. The equation of motion is dv mg Ϫ bv ϭ m 3-10 dt where b is given by Stokes’ law: b ϭ 6a 3-11 and where is the coefficient of viscosity of the fluid (air) and a is the radius of the drop. The terminal velocity of the falling drop vf is Atomizer (+) (–) Light source (–) (+) Telescope Fig. 3-4 Schematic diagram of the Millikan oil-drop apparatus. The drops are sprayed from the atomizer and pick up a static charge, a few falling through the hole in the top plate. Their fall due to gravity and their rise due to the electric field between the capacitor plates can be observed with the telescope. From measurements of the rise and fall times, the electric charge on a drop can be calculated. The charge on a drop could be changed by exposure to x rays from a source (not shown) mounted opposite the light source. (Continued) 12 More mg Buoyant force bv v ϭ 3-12 f b (see Figure 3-5). When an electric field Ᏹ is applied, the upward motion of a charge Droplet e qn is given by dv Weight mg q Ᏹ Ϫ mg Ϫ bv ϭ m n dt Fig. -

Millikan Oil Drop Experiment
Agenda 1. Measuring of the charge of electron. 2. Robert Millikan and his oil drop experiment 3. Theory of the experiment 4. Laboratory setup 5. Data analysis 2/17/2014 2 Measuring of the charge of the electron 1. Oil drop experiment. Robert A. Millikan.. (1909). e=1.5924(17)×10−19 C 2. Shot noise experiment. First proposed by Walter H. Schottky 3. In terms of the Avogadro constant and Faraday constant 풆 = 푭 ; F- Faraday constant, NA - Avagadro constant. Best 푵푨 uncertainty ~1.6 ppm. ퟐ풆 풉 4. From Josephson (푲 = ) and von Klitzing 푹 = constants 푱 풉 푲 풆ퟐ 5. Recommended by NIST value 1.602 176 565(35) 10-19 C 2/17/2014 3 Robert Millikan. Oil drop experiment The Nobel Prize in Physics 1923. Robert A. Millikan "for his work on the elementary charge of electricity and on the photoelectric effect". ROBERT ANDREWS MILLIKAN 1868-1953 22nd of March, 1868, Morrison, Ill University of Chicago 2/17/2014 4 Robert Millikan. Oil drop experiment ROBERT ANDREWS MILLIKAN 1868-1953 Diagram and picture of apparatus 2/17/2014 5 Oil drop experiment. Measurement of the magnitude of the electron charge! Motivation: Demonstrate that the electron charge is quantized! Measure the charge of an electron to ±3% Picture of the PASCO setup 2/17/2014 6 Oil drop experiment. atomizer Oil drops ∅~1m telescope + V Vg d 500V - 흆풂풊풓 Forces on the oil drop: 1) Gravity + buoyant force (air displaced by oil drop) 2) Drag force of the oil drop in the air 2/17/2014 7 Oil drop experiment. -

Chronological List of Correspondence, 1895–1920
CHRONOLOGICAL LIST OF CORRESPONDENCE, 1895–1920 In this chronological list of correspondence, the volume and document numbers follow each name. Documents abstracted in the calendars are listed in the Alphabetical List of Texts in this volume. 1895 13 or 20 Mar To Mileva Maric;;, 1, 45 29 Apr To Rosa Winteler, 1, 46 Summer To Caesar Koch, 1, 6 18 May To Rosa Winteler, 1, 47 28 Jul To Julia Niggli, 1, 48 Aug To Rosa Winteler, 5: Vol. 1, 48a 1896 early Aug To Mileva Maric;;, 1, 50 6? Aug To Julia Niggli, 1, 51 21 Apr To Marie Winteler, with a 10? Aug To Mileva Maric;;, 1, 52 postscript by Pauline Einstein, after 10 Aug–before 10 Sep 1,18 From Mileva Maric;;, 1, 53 7 Sep To the Department of Education, 10 Sep To Mileva Maric;;, 1, 54 Canton of Aargau, 1, 20 11 Sep To Julia Niggli, 1, 55 4–25 Nov From Marie Winteler, 1, 29 11 Sep To Pauline Winteler, 1, 56 30 Nov From Marie Winteler, 1, 30 28? Sep To Mileva Maric;;, 1, 57 10 Oct To Mileva Maric;;, 1, 58 1897 19 Oct To the Swiss Federal Council, 1, 60 May? To Pauline Winteler, 1, 34 1900 21 May To Pauline Winteler, 5: Vol. 1, 34a 7 Jun To Pauline Winteler, 1, 35 ? From Mileva Maric;;, 1, 61 after 20 Oct From Mileva Maric;;, 1, 36 28 Feb To the Swiss Department of Foreign Affairs, 1, 62 1898 26 Jun To the Zurich City Council, 1, 65 29? Jul To Mileva Maric;;, 1, 68 ? To Maja Einstein, 1, 38 1 Aug To Mileva Maric;;, 1, 69 2 Jan To Mileva Maric;; [envelope only], 1 6 Aug To Mileva Maric;;, 1, 70 13 Jan To Maja Einstein, 8: Vol. -

Spectroscopy & the Nobel
Newsroom 1971 CHEMISTRY NOBEL OSA Honorary Member Gerhard Herzberg “for his contributions to the knowledge of electronic structure and geometry of molecules, particularly free radicals” 1907 PHYSICS NOBEL 1930 PHYSICS NOBEL 1966 CHEMISTRY NOBEL OSA Honorary Member Albert OSA Honorary Member Sir Robert S. Mulliken “for Abraham Michelson “for his Chandrasekhara Venkata his fundamental work optical precision instruments Raman “for his work on the concerning chemical bonds and the spectroscopic and scattering of light and for and the electronic structure metrological investigations the discovery of the effect of molecules by the carried out with their aid” named after him” molecular orbital method” 1902 PHYSICS NOBEL 1919 PHYSICS NOBEL Hendrik Antoon Lorentz and Johannes Stark “for his Pieter Zeeman “for their discovery of the Doppler researches into the influence effect in canal rays and of magnetism upon radiation the splitting of spectral phenomena” lines in electric fields” 1955 PHYSICS NOBEL OSA Honorary Member Willis Eugene Lamb “for his discoveries concerning the fine structure of the hydrogen Spectroscopy spectrum” & the Nobel ctober is when scientists around the world await the results from Stockholm. O Since the Nobel Prize was established in 1895, a surprising number of the awards have gone to advances related to or enabled by spectroscopy—from the spectral splitting of the Zeeman and Stark effects to cutting-edge advances enabled by laser frequency combs. We offer a small (and far from complete) sample here; to explore further, visit www.nobelprize.org. 16 OPTICS & PHOTONICS NEWS OCTOBER 2018 1996 CHEMISTRY NOBEL OSA Fellow Robert F. Curl Jr., Richard Smalley and Harold 1999 CHEMISTRY NOBEL Kroto (not pictured) “for their Ahmed H. -

Historical Background Prepared by Dr, Robin Chaplin Professor of Power Plant Engineering (Retired) University of New Brunswick
1 Historical Background prepared by Dr, Robin Chaplin Professor of Power Plant Engineering (retired) University of New Brunswick Summary: A review of the historical background for the development of nuclear energy is given to set the scene for the discussion of CANDU reactors. Table of Contents 1 Growth of Science and Technology......................................................................................... 2 2 Renowned Scientists............................................................................................................... 4 3 Significant Achievements........................................................................................................ 6 3.1 Niels Bohr........................................................................................................................ 6 3.2 James Chadwick .............................................................................................................. 6 3.3 Enrico Fermi .................................................................................................................... 6 4 Nuclear Fission........................................................................................................................ 7 5 Nuclear Energy........................................................................................................................ 7 6 Acknowledgments................................................................................................................... 8 List of Figures Figure 1 Timeline of significant discoveries -

In Defense of Robert Andrews Millikan by David Goodstein
30 ENGINEERING & SCIENCE NO. 4 He also has been accused of male chauvinism, anti-Semitism, mistreating his graduate students, and, worst of all, scientific fraud. In Defense of Robert Andrews Millikan by David Goodstein Robert Andrews Millikan was the founder, first Thomson and his colleagues tried to do it by leader, first Nobel Prize winner and all-around observing how an applied electric field changed patron saint of the California Institute of Technol- the rate of gravitational fall of clouds of water ogy, an institution that has given me employment droplets that had nucleated on ions in a cloud for more years than I care to remember. He also chamber. The upper edge of the cloud, which had has been accused of male chauvinism, anti- the smallest droplets, could be assumed to contain Semitism, mistreating his graduate students, and, single charges. In this way, a crude but correct worst of all, scientific fraud. Since we at Caltech estimate of the unit of electric charge could be feel a solemn duty to defend our hero, my purpose obtained. These cloud-chamber experiments were Isaac Newton (framed) and here is to tell his story, look into these various the starting point of Millikan’s efforts. accusations, and, to the extent that I can, mount Working with a graduate student named Louis Robert Millikan: colleagues a defense for Professor Millikan. Begeman, Millikan had the idea of applying a in crime? Speaking of Millikan was born in 1868, son of a Midwestern much stronger electric field than had previously crime, the Newton portrait minister. -

A Debate on Magnetic Current: the Troubled Einstein–Ehrenhaft Correspondence
BJHS 44(3): 371–400, September 2011. © British Society for the History of Science 2010 doi:10.1017/S0007087410001299 First published online 8 October 2010 A debate on magnetic current: the troubled Einstein–Ehrenhaft correspondence GILDO MAGALHÃES SANTOS* Abstract. The unconventional correspondence between physicists Albert Einstein and Felix Ehrenhaft, especially at the height of the alleged production by the latter of magnetic monopoles, is examined in the following paper. Almost unknown by the general public, it is sometimes witty, yet it can be pathetic, and certainly bewildering. At one point the arguments they exchanged became a poetic duel between Einstein and Ehrenhaft’s wife. Ignored by conventional Einstein biographies, this episode took place during the initial years of the Second World War, but was rooted in disputes dating back to the early years of the twentieth century. The interesting intersection of a series of scientific controversies also highlights some aspects of the personal dramas involved, and after so many years the whole affair in itself is still intriguing. The issues in the Ehrenhaft–Einstein epistolary Science has now practically forgotten the polemic figure of Felix Albert Ehrenhaft (1879–1952), an Austrian physicist who in the 1900s and 1910s assumed the existence of electric charges smaller than the electron, based on his experimental work. Three decades later, Ehrenhaft came up with what appeared to be another heresy, insisting that he had observed isolated magnetic poles. He maintained a correspondence with Albert Einstein on these subjects for about thirty years, trying to convince Einstein of the validity of his arguments, while Einstein attacked Ehrenhaft’s conclusions, but followed his experimental work. -
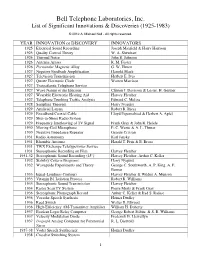
Btl Innovation List
Bell Telephone Laboratories, Inc. List of Significant Innovations & Discoveries (1925-1983) © 2012 A. Michael Noll - All rights reserved. YEAR INNOVATION or DISCOVERY INNOVATORS 1925 Electrical Sound Recording Joseph Maxfield & Harry Harrison 1925 Quality Control Theory W. A. Shewhart 1926 Thermal Noise John B. Johnson 1926 Antenna Arrays R. M. Foster 1926 Permendur Magnetic Alloy G. W. Elmen 1927 Negative Feedback Amplification Harrold Black 1927 Television Transmission Herbert E. Ives 1927 Quartz Electronic Clock Warren Marrison 1927 Transatlantic Telephone Service 1927 Wave Nature of the Electron Clinton J. Davisson & Lester. H. Germer 1927 Wearable Electronic Hearing Aid Harvey Fletcher 1927 Telephone Trunking Traffic Analysis Edward C. Molina 1928 Sampling Theorem Harry Nyquist 1929 Artificial Larynx Robert R. Riesz 1929 Broadband Coaxial Cable Lloyd Espenschied & Herbert A. Apfel 1929 Ship-to-Shore Radio System 1929 Frequency Interleaving of TV Signal Frank Gray & John R. Hefele 1930 Moving-Coil Microphone E. C. Wente & A. L. Thuras 1930 Negative Impedance Repeater George Crisson 1931 Radio Astronomy Karl Jansky 1931 Rhombic Antenna Harald T. Friis & E. Bruce 1931 TWX Exchange Teletypewriter Service 1931 Stereophonic Recording on Film Harvey Fletcher 1931-32 Stereophonic Sound Recording (45°) Harvey Fletcher, Arthur C. Keller 1932 Stability Criteria Diagrams Harry Nyquist 1932 Waveguide Experiments and Theory George C. Southworth, A. P. King, A. E. Bowen 1933 Equal-Loudness Contours Harvey Fletcher & Wilden A. Munson 1933 Vitamin B1 Isolation Process Robert R. Williams 1933 Stereophonic Sound Transmission Harvey Fletcher 1934 Raster Scan TV System Pierre Mertz & Frank Gray 1936 Stereophonc Phonograph Record Arthur C. Keller & Irad S. Rafuse 1936 Vocoder Speech Synthesis Homer Dudley 1936 Reed Switch Walter B.