Neurogenic Lower Urinary Tract Dysfunction
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Urinary Incontinence: Impact on Long Term Care
Urinary Incontinence: Impact on Long Term Care Muhammad S. Choudhury, MD, FACS Professor and Chairman Department of Urology New York Medical College Director of Urology Westchester Medical Center 1 Urinary Incontinence: Overview • Definition • Scope • Anatomy and Physiology of Micturition • Types • Diagnosis • Management • Impact on Long Term Care 2 Urinary Incontinence: Definition • Involuntary leakage of urine which is personally and socially unacceptable to an individual. • It is a multifactorial syndrome caused by a combination of: • Genito urinary pathology. • Age related changes. • Comorbid conditions that impair normal micturition. • Loss of functional ability to toilet oneself. 3 Urinary Incontinence: Scope • Prevalence of Urinary incontinence increase with age. • Affects more women than men (2:1) up to age 80. • After age 80, both women and men are equally affected. • Urinary Incontinence affect 15% to 30% of the general population > 65 years. • > 50% of 1.5 million Long Term Care residents may be incontinent. • The cost to care for this group is >5 billion per year. • The total cost of care for Urinary Incontinence in the U.S. is estimated to be over $36 billion. Ehtman et al., 2012. 4 Urinary Incontinence: Impact on Quality of Life • Loss of self esteem. • Avoidance of social activity and interaction. • Decreased ability to maintain independent life style. • Increased dependence on care givers. • One of the most common reason for long term care placement. Grindley et al. Age Aging. 1998; 22: 82-89/Harris T. Aging in the eighties. NCHS # 121 1985. Noelker L. Gerontologist 1987; 27: 194-200. 5 Health related consequences of Urinary Incontinence • Increased propensity for fall/fracture. -

Urinary Incontinence
GLICKMAN UROLOGICAL & KIDNEY INSTITUTE Urinary Incontinence What is it? can lead to incontinence, as can prostate cancer surgery or Urinary incontinence is the inability to control when you radiation treatments. Sometimes the cause of incontinence pass urine. It’s a common medical problem. As many as isn’t clear. 20 million Americans suffer from loss of bladder control. The condition is more common as men get older, but it’s Where can I get help? not an inevitable part of aging. Often, embarrassment stops Talking to your doctor is the first step. You shouldn’t feel men from seeking help, even when the problem is severe ashamed; physicians regularly help patients with this prob- and affects their ability to leave the house, spend time with lem and are comfortable talking about it. Many patients family and friends or take part in everyday activities. It’s can be evaluated and treated after a simple office visit. possible to cure or significantly improve urinary inconti- Some patients may require additional diagnostic tests, nence, once its underlying cause has been identified. But which can be done in an outpatient setting and aren’t pain- it’s important to remember that incontinence is a symp- ful. Once these tests have determined the cause of your tom, not a disease. Its cause can be complex and involve incontinence, your doctor can recommend specific treat- many factors. Your doctor should do an in-depth evaluation ments, many of which do not require surgery. No matter before starting treatment. how serious the problem seems, urinary incontinence is a condition that can be significantly relieved and, in many What might be causing my incontinence? cases, cured. -

The National Drugs List
^ ^ ^ ^ ^[ ^ The National Drugs List Of Syrian Arab Republic Sexth Edition 2006 ! " # "$ % &'() " # * +$, -. / & 0 /+12 3 4" 5 "$ . "$ 67"5,) 0 " /! !2 4? @ % 88 9 3: " # "$ ;+<=2 – G# H H2 I) – 6( – 65 : A B C "5 : , D )* . J!* HK"3 H"$ T ) 4 B K<) +$ LMA N O 3 4P<B &Q / RS ) H< C4VH /430 / 1988 V W* < C A GQ ") 4V / 1000 / C4VH /820 / 2001 V XX K<# C ,V /500 / 1992 V "!X V /946 / 2004 V Z < C V /914 / 2003 V ) < ] +$, [2 / ,) @# @ S%Q2 J"= [ &<\ @ +$ LMA 1 O \ . S X '( ^ & M_ `AB @ &' 3 4" + @ V= 4 )\ " : N " # "$ 6 ) G" 3Q + a C G /<"B d3: C K7 e , fM 4 Q b"$ " < $\ c"7: 5) G . HHH3Q J # Hg ' V"h 6< G* H5 !" # $%" & $' ,* ( )* + 2 ا اوا ادو +% 5 j 2 i1 6 B J' 6<X " 6"[ i2 "$ "< * i3 10 6 i4 11 6! ^ i5 13 6<X "!# * i6 15 7 G!, 6 - k 24"$d dl ?K V *4V h 63[46 ' i8 19 Adl 20 "( 2 i9 20 G Q) 6 i10 20 a 6 m[, 6 i11 21 ?K V $n i12 21 "% * i13 23 b+ 6 i14 23 oe C * i15 24 !, 2 6\ i16 25 C V pq * i17 26 ( S 6) 1, ++ &"r i19 3 +% 27 G 6 ""% i19 28 ^ Ks 2 i20 31 % Ks 2 i21 32 s * i22 35 " " * i23 37 "$ * i24 38 6" i25 39 V t h Gu* v!* 2 i26 39 ( 2 i27 40 B w< Ks 2 i28 40 d C &"r i29 42 "' 6 i30 42 " * i31 42 ":< * i32 5 ./ 0" -33 4 : ANAESTHETICS $ 1 2 -1 :GENERAL ANAESTHETICS AND OXYGEN 4 $1 2 2- ATRACURIUM BESYLATE DROPERIDOL ETHER FENTANYL HALOTHANE ISOFLURANE KETAMINE HCL NITROUS OXIDE OXYGEN PROPOFOL REMIFENTANIL SEVOFLURANE SUFENTANIL THIOPENTAL :LOCAL ANAESTHETICS !67$1 2 -5 AMYLEINE HCL=AMYLOCAINE ARTICAINE BENZOCAINE BUPIVACAINE CINCHOCAINE LIDOCAINE MEPIVACAINE OXETHAZAINE PRAMOXINE PRILOCAINE PREOPERATIVE MEDICATION & SEDATION FOR 9*: ;< " 2 -8 : : SHORT -TERM PROCEDURES ATROPINE DIAZEPAM INJ. -

CMS Manual System Human Services (DHHS) Pub
Department of Health & CMS Manual System Human Services (DHHS) Pub. 100-07 State Operations Centers for Medicare & Provider Certification Medicaid Services (CMS) Transmittal 8 Date: JUNE 28, 2005 NOTE: Transmittal 7, of the State Operations Manual, Pub. 100-07 dated June 27, 2005, has been rescinded and replaced with Transmittal 8, dated June 28, 2005. The word “wound” was misspelled in the Interpretive Guidance section. All other material in this instruction remains the same. SUBJECT: Revision of Appendix PP – Section 483.25(d) – Urinary Incontinence, Tags F315 and F316 I. SUMMARY OF CHANGES: Current Guidance to Surveyors is entirely replaced by the attached revision. The two tags are being combined as one, which will become F315. Tag F316 will be deleted. The regulatory text for both tags will be combined, followed by this revised guidance. NEW/REVISED MATERIAL - EFFECTIVE DATE*: June 28, 2005 IMPLEMENTATION DATE: June 28, 2005 Disclaimer for manual changes only: The revision date and transmittal number apply to the red italicized material only. Any other material was previously published and remains unchanged. However, if this revision contains a table of contents, you will receive the new/revised information only, and not the entire table of contents. II. CHANGES IN MANUAL INSTRUCTIONS: (N/A if manual not updated.) (R = REVISED, N = NEW, D = DELETED) – (Only One Per Row.) R/N/D CHAPTER/SECTION/SUBSECTION/TITLE R Appendix PP/Tag F315/Guidance to Surveyors – Urinary Incontinence D Appendix PP/Tag F316/Urinary Incontinence III. FUNDING: Medicare contractors shall implement these instructions within their current operating budgets. IV. ATTACHMENTS: Business Requirements x Manual Instruction Confidential Requirements One-Time Notification Recurring Update Notification *Unless otherwise specified, the effective date is the date of service. -

Autonomic Nervous System Dysfunction Involving the Gastrointestinal and the Urinary Tracts in Primary Sjögren’S Syndrome
Autonomic nervous system dysfunction involving the gastrointestinal and the urinary tracts in primary Sjögren’s syndrome L. Kovács1, M. Papós2, R. Takács3, R. Róka2, Z. Csenke4, A. Kovács1, T. Várkonyi3, L. Pajor5, L. Pávics2, G. Pokorny1 Department of Rheumatology1, Department of Nuclear Medicine2, 1st Department of Internal Medicine3 and Department of Urology5, University of Szeged, Faculty of Medicine, Szeged; Division of Urology, Municipial Clinic, Szeged, Hungary4 Abstract Objective Antibodies reacting with the m3 subtype muscarinic acetylcholine receptor appear to be an important patho- genic factor in primary Sjögren’s syndrome (pSS). As this receptor subtype is functionally important in the gastrointestinal and urinary tracts, and very little is known about the autonomic nervous system function in these organs in pSS patients, the occurrence and clinical significance of an autonomic nervous system dysfunction involving the gastrointestinal and urinary tracts were investigated. Methods Data on clinical symptoms attributable to an autonomic dysfunction were collected from 51 pSS patients. Gastric emptying scintigraphy and urodynamic studies were performed on 30 and 16 patients, respectively, and the results were correlated with patient characteristics and with the presence of autonomic nervous system symptoms. Results Gastric emptying was abnormally slow in 21 of the 30 examined patients (70%). Urodynamic findings compatible with a decreased detrusor muscle tone or contractility were found in 9 of the 16 patients tested (56%). Various symptoms of an autonomic nervous system dysfunction were reported by 2-16% of the patients. Conclusion Signs of an autonomic nervous system dysfunction involving the gastrointestinal and the urinary systems can be observed in the majority of pSS patients. -

Urinary Tract Infection (Uti) Fact Sheet
URINARY TRACT INFECTION (UTI) FACT SHEET Overview A urinary tract infection (UTI) is an infection that involves any part of the urinary tract, including the kidneys, bladder and urethra. It is usually caused by exposure of the urinary tract to a fecal organism such as Escherichia coli (E. coli) but also may be caused by other organisms. An indwelling urinary catheter is a drainage tube that is inserted into the urinary bladder through the urethra, is left in place and is connected to a closed urine collection system. Urinary tract infections in patients with an indwelling urinary catheter are called catheter-associated UTIs, or CAUTIs. Signs and Symptoms Common symptoms of UTI include: • fever; • pain or burning in the lower abdomen; • burning during urination; • an increase in the frequency of urination; and • cloudy appearing urine. Causes and Transmission A UTI occurs when germs (usually bacteria) enter the urinary tract through the meatus (the opening of the urinary tract). These germs then cause infection. The urinary tract is normally sterile, meaning it contains no germs. A CAUTI occurs when germs (usually bacteria) enter the urinary tract through the urinary catheter and cause infection. This can occur when health care worker hands are not properly cleaned before the insertion of a catheter or during the process of cleaning or emptying of the urine collection system. Risk Factors Sexually active women, people with blockages in the urinary tract, such as prostate enlargement, and women using certain types of birth control, such as diaphragms, or those who are undergoing menopause are at higher risk of UTI. -

Urinary Incontinence Embarrassing but Treatable 2015 Rev
This information provides a general overview on this topic and may not apply to Health Notes everyone. To find out if this information applies to you and to get more information on From Your Family Doctor this subject, talk to your family doctor. Urinary incontinence Embarrassing but treatable 2015 rev. What is urinary incontinence? Are there different types Urinary incontinence means that you can’t always of incontinence? control when you urinate, or pee. The amount of leakage Yes. There are five types of urinary incontinence. can be small—when you sneeze, cough, or laugh—or large, due to very strong urges to urinate that are hard to Stress incontinence is when urine leaks because of control. This can be embarrassing, but it can be treated. sudden pressure on your lower stomach muscles, such as when you cough, sneeze, laugh, rise from a Millions of adults in North America have urinary chair, lift something, or exercise. Stress incontinence incontinence. It’s most common in women over 50 years usually occurs when the pelvic muscles are weakened, of age, but it can also affect younger people, especially sometimes by childbirth, or by prostate or other pelvic women who have just given birth. surgery. Stress incontinence is common in women. Be sure to talk to your doctor if you have this problem. Urge incontinence is when the need to urinate comes on If you hide your incontinence, you risk getting rashes, too fast—before you can get to a toilet. Your body may only sores, and skin and urinary tract (bladder) infections. -

Updatirg the Beers Criteria for Potentially Inappropriate Medication Use in Older Adults
Updatirg the BeersCriteria for Potentially InappropriateMedication Use in Older Adults Resultsof a US ConsensusPanel of Experts DonnaM.Fich,PhD,RN;lamesW.Cooper,PhD,RPh;WilliamE.Wade,PhannD,FASHP,FCCP; JenniJerL. Waller, PhD;J, RossMaclean, MD; Marh H. Beers,MD Bcckground: Medication toxic effectsand drug- Reruhr: This study identified 48 individual medica- relatedproblems can have profound medical and safety tions or classeso[ medicationsto avoid in older adults consequencesfor older adults and economically affect the and their potential concernsand 20 diseases/conditions health caresystem. The purpose of this initiative was to and medicationsto be avoidedin older adultswith these reviseand update the Beerscriteria for potentially inap- conditions.Of thesepotentially inappropriate drugs, 66 propriate medicationuse in adults 65 yearsand older in wereconsidered by the panelto haveadverse outcomes the United States. of high severity. lYlcthcdr: This study used a modified Delphi method, a Concludonr: This study is an importantupdate of pre- setof proceduresand methodsfor formulating a groupjudg- viously establishedcriteria that have been widely used ment for a subject matter in which precise information is and cited. The application of the Beerscriteria and other Iacking. The criteria reviewed covered 2 types of state- tools for identifying potentially inapproprlate medica- ments: (l) medicationsor medicationclasses that should tion use will continue to enableproviders to plan inter- grnerally be avoidedin persons 65 years or older because -

Acute Onset Flank Pain-Suspicion of Stone Disease (Urolithiasis)
Date of origin: 1995 Last review date: 2015 American College of Radiology ® ACR Appropriateness Criteria Clinical Condition: Acute Onset Flank Pain—Suspicion of Stone Disease (Urolithiasis) Variant 1: Suspicion of stone disease. Radiologic Procedure Rating Comments RRL* CT abdomen and pelvis without IV 8 Reduced-dose techniques are preferred. contrast ☢☢☢ This procedure is indicated if CT without contrast does not explain pain or reveals CT abdomen and pelvis without and with 6 an abnormality that should be further IV contrast ☢☢☢☢ assessed with contrast (eg, stone versus phleboliths). US color Doppler kidneys and bladder 6 O retroperitoneal Radiography intravenous urography 4 ☢☢☢ MRI abdomen and pelvis without IV 4 MR urography. O contrast MRI abdomen and pelvis without and with 4 MR urography. O IV contrast This procedure can be performed with US X-ray abdomen and pelvis (KUB) 3 as an alternative to NCCT. ☢☢ CT abdomen and pelvis with IV contrast 2 ☢☢☢ *Relative Rating Scale: 1,2,3 Usually not appropriate; 4,5,6 May be appropriate; 7,8,9 Usually appropriate Radiation Level Variant 2: Recurrent symptoms of stone disease. Radiologic Procedure Rating Comments RRL* CT abdomen and pelvis without IV 7 Reduced-dose techniques are preferred. contrast ☢☢☢ This procedure is indicated in an emergent setting for acute management to evaluate for hydronephrosis. For planning and US color Doppler kidneys and bladder 7 intervention, US is generally not adequate O retroperitoneal and CT is complementary as CT more accurately characterizes stone size and location. This procedure is indicated if CT without contrast does not explain pain or reveals CT abdomen and pelvis without and with 6 an abnormality that should be further IV contrast ☢☢☢☢ assessed with contrast (eg, stone versus phleboliths). -
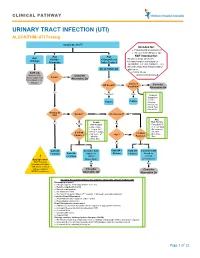
URINARY TRACT INFECTION (UTI) ALGORITHM- UTI Testing
CLINICAL PATHWAY URINARY TRACT INFECTION (UTI) ALGORITHM- UTI Testing Suspicion of UTI Intended for: • Patients with presumed UTI • Greater than 60days of age Age Age NOT intended for: Age 60days- >36months or • Known urologic anomalies <60days • 36months Toilet Trained Chronic/complex conditions (ie. spinabifida, self cath, hardware, etc.) • Recent urinary tract instrumentation Clean Catch UA placement Cath UA • Critical Illness Refer to CCG Consider • Immunocompromised Fever? NO “Fever, infant (less Alternative Dx than 28days or 28- 90days)” Index of UA Result? Neg Low Consider Suspicion* Alternative Dx Yes Pos/Equiv High *Index of Suspicion • Febrile Culture Culture • Dysuria • Frequency • Flank Pain • Hx of UTI History of No Gender? Male Circumcised? YES UTI? Male Female Risk Factors Yes NO Female Risk Factors • Temp ≥ 39°C • Age <12mo • Fever ≥2 days • Temp ≥ 39°C • No source of • ≥ 3 Risk Fever ≥ 2 days infection • No source of ≥ 3 Risk • Non-black Factors? <1yr? No infection Factors? Race • White Race Yes Yes No Yes No Cath UA Consider Cath Cath UA + Cath UA Consider Cath + Culture Cath UA based on Culture + Culture based on ! + Culture clinical clinical Bag Specimen presentation presentation NOT Preferred Neg Neg (consider with labial adhesions, or failed catheterizations) Consider Consider NEVER send culture Alternative Dx Alternative Dx Imaging Recommendations for patients >2months after 1st Febrile UTI No imaging required o Prompt response to therapy (afebrile in 72 hrs) o Reliable outpatient follow up o Normal voiding pattern -

STRESS INCONTINENCE by JOHN BEATTIE, M.D., F.R.C.S., F.R.C.O.G
Postgrad Med J: first published as 10.1136/pgmj.32.373.527 on 1 November 1956. Downloaded from s27 STRESS INCONTINENCE By JOHN BEATTIE, M.D., F.R.C.S., F.R.C.O.G. Gynaecological Surgeon, St. Bartholomew's Hospital So far the mist has cleared very little from around obtain such a history, otherwise a patient may be this difficult subject despite the original work operated upon for urethrocele when the symptoms which has been carried out during the last io are due to something else or if true stress incon- years. Operations for the cure of stress incon- tinence develops after child bearing in such a tinence have been described from time to time patient the results of operation may be dis- during the last 50 years, but until recently most appointing. gynaecologists considered that a modified anterior Other abnormalities of bladder function which colporrhaphy was all that was necessary to achieve must be differentiated from a case of true stress a lasting cure. incontinence are as follows: One of the most important things in dealing with this subject is first to differentiate true stress The Neurogenic Bladder incontinence from inability to control the passage The bladder is often a most sensitive indicator Protected by copyright. of urine from the bladder when the urge to mic- of neurological disease and, of course, there may turate becomes great. There is also the leak which be, and often is, vaginal prolapse present at the occurs in retention with overflow to consider and onset of such a condition. The most often quoted other conditions which cause weakness of the instance of the neurogenic bladder is disseminated bladder sphincterwithout a true stress incontinence sclerosis, and, indeed, it is the commonest neuro- being present. -

Peripheral Neuropathy Diagnostic Services
Peripheral Neuropathy Diagnostic Services Approximately 1 out of every 15 people (20 million) suffer from Neuropathy and more than 30% of all Neuropathies stem from Diabetes. Digirad provides Peripheral Neuropathy diagnostic services to healthcare providers that treat patients who are at risk from autonomic dysfunction or autonomic neuropathy. Adding these services is convenient for your patients and provides a new revenue stream for your practice. Program Overview The Peripheral Neuropathy Diagnostics service is performed by our technologist in a standard patient exam room. The patient’s peripheral nervous system is monitored and evaluated for its response to stimulation. The test is relatively quick, pain free and patient satisfaction is very high. After the test is performed, a detailed set of results and analysis are delivered back to you. The results provide a clear understanding of the presence, and extent of, peripheral autonomic dysfunction or disease. Additional studies may be performed to identify the cause of symptoms such as numbness, tingling, continuous pain and the early detection of diabetes complications. How it Works • The program is overseen by highly qualified Neurologists • One of our highly trained technologists will arrive on your scheduled date to perform testing • We provide the state-of-the-art equipment and tests are done in one of your exam rooms • Reports are delivered within 24-48 hours using Digirad’s digital delivery system • This system permanently stores patients’ test results allowing you unlimited access Contact us to learn more: 855-644-3443 | www.digirad.com Digirad delivers diagnostic imaging expertise. As Needed. When Needed. Where Needed. Autonomic Neuropathy Autonomic neuropathy is a disorder that affects involuntary body functions, including heart rate, blood pressure, perspiration and digestion.