Response to Disturbance Abstract and Keywords Key Points
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Molluscs and Crustaceans of Glasgow Botanic Gardens, Scotland
The Glasgow Naturalist (online 2020) Volume 27, Part 3, 93-95 https://doi.org/10.37208/tgn27317 *Melanoides tuberculata (O.F. Müller, 1774). Red-rimmed melania. In a tropical pond in the Lily The molluscs and crustaceans of House (TW). There were thin and truncated Melanoides specimens found in the same pond and in the pond in the Glasgow Botanic Gardens, Scotland Orchid House (TW), but it is safe to assume that they too are M. tuberculata as it is a very variable species R.B. Weddle (BR) Arionidae 89 Novar Drive, Glasgow G12 9SS Arion owenii Davies, 1979. Tawny soil slug (AS). Arion rufus (Linnaeus, 1758). Large red slug (AS). E-mail: [email protected] Arion subfuscus (Draparnaud, 1805). Dusky slug. Main Gardens and North Kelvin area (AS; J. Dempster, 2018). Carychiinae This note focuses on mollusc and crustacean species that Carychium minimum O.F. Müller, 1774. Short- are additional to those listed as present in Glasgow toothed herald snail. Kibble Palace (TW). Botanic Gardens by Hancock (1999). Helicidae Cepaea hortensis (O.F. Müller, 1774). White-lipped MOLLUSCA snail. Arboretum (AS); Main Gardens (A. Malcolm, Since Hancock’s original On the Wildside account 2015). (Hancock, 1999) there have been several visits to the Cepaea nemoralis (Linnaeus, 1758). Brown-lipped Gardens, particularly to the glasshouses, by specialist snail. North Kelvin area (R.B. Weddle, 2011); Main conchologists, and several bioblitzes. This note Gardens summarises the recent findings and reviews one of the (A. Malcolm, 2018) historical records mentioned by Hancock. The absence of both Cepaea species from Hancock’s list is puzzling since there are records in Glasgow generally Nineteen species have been added to Hancock’s list, since the late 19th century (Glasgow Museums BRC). -

Singapore Biodiversity Records Xxxx: Xx
SINGAPORE BIODIVERSITY RECORDS 2019: 18 ISSN 2345-7597 Date of publication: 28 February 2019. © National University of Singapore Confirmed occurrence of the awl snail, Striosubulina striatella, in Singapore Chan Sow-Yan [email protected] __________________________________________________________________________________________ Subjects: Awl snail, Striosubulina striatella (Mollusca: Gastropoda: Achatinidae). Subjects identified by: Chan Sow-Yan. Location, date and time: Singapore Island, Mei Hwan Drive; 11 January 2019; late afternoon. Habitat: Urban parkland. In playground, at the base of a tembusu tree. Observer: Chan Sow-Yan. Observation: At least three examples were seen exposed among leaf debris in the shade of a tembusu tree after light rain. One example, of about 10.2 mm shell length, is illustrated in Fig. 1. They were found together with the morphologically similar Subulina octona (Fig. 2). Fig. 1. Dorsal view of Striosubulina striatella showing Fig. 2. Dorsal view of Subulina octona showing its strong axial sculpture and dark mark on its eyestalk. smoother axial sculpture. Photographs by Chan Sow Yan Remarks: Striosubulina striatella was first recorded in Singapore as ‘? Subulina striatella’ with doubt by Maassen (2001). The species was described as Helix striatella from Principe Island in the Gulf of Guinea, West Africa by Rang (1831). It was recently introduced to East Africa (Rowson et al., 2010) among many other places. This snail is apparently not native to Singapore, and likely to have been introduced there earlier than Maassen’s record in 2001. It seems to have escaped detection due to its strong resemblance in both appearance and size to the locally abundant con-familial Subulina octona. Although both species have a similar truncated columella, Striosubulina striatella can be distinguished by its fine axial sculpture (if observed under a X10 hand lens), as well as a faint dark area on its eye stalks (see Fig. -

Conchological Differentiation and Genital Anatomy of Nepalese Glessulinae (Gastropoda, Stylommatophora, Subulinidae), with Descriptions of Six New Species
A peer-reviewed open-access journal ZooKeys 675: 129–156Conchological (2017) differentiation and genital anatomy of Nepalese Glessulinae... 129 doi: 10.3897/zookeys.675.13252 RESEARCH ARTICLE http://zookeys.pensoft.net Launched to accelerate biodiversity research Conchological differentiation and genital anatomy of Nepalese Glessulinae (Gastropoda, Stylommatophora, Subulinidae), with descriptions of six new species Prem B. Budha1,3, Fred Naggs2, Thierry Backeljau1,4 1 University of Antwerp, Evolutionary Ecology Group, Universiteitsplein 1, B-2610, Antwerp, Belgium 2 Na- tural History Museum, Cromwell Road, London, SW7 5BD, UK 3 Central Department of Zoology, Tribhuvan University, Kirtipur, Kathmandu, Nepal 4 Royal Belgian Institute of Natural Sciences, Vautierstraat 29, B-1000, Brussels, Belgium Corresponding author: Prem B. Budha ([email protected]) Academic editor: F. Köhler | Received 17 April 2017 | Accepted 2 May 2017 | Published 23 May 2017 http://zoobank.org/E5C8F163-D615-47B9-8418-CEE8D71A7DAB Citation: Budha PB, Naggs F, Backeljau T (2017) Conchological differentiation and genital anatomy of Nepalese Glessulinae (Gastropoda, Stylommatophora, Subulinidae), with descriptions of six new species. ZooKeys 675: 129– 156. https://doi.org/10.3897/zookeys.675.13252 Abstract Eleven species of Glessulinae belonging to the genera Glessula Martens, 1860 (three species) and Rishetia Godwin-Austen, 1920 (eight species) are reported from Nepal, six of which are new to science and are described here, viz., G. tamakoshi Budha & Backeljau, sp. n., R. kathmandica Budha & Backeljau, sp. n., R. nagarjunensis Budha & Naggs, sp. n., R. rishikeshi Budha & Naggs, sp. n., R. subulata Budha & Naggs and R. tribhuvana Budha, sp. n. and two are new records for Nepal viz. G. cf. hebetata and R. -

The Land and Fresh-Water Mollusks of Puerto Rico
MISCELLANEOUS PUBLICATIONS MUSEUM OF ZOOLOGY, UNIVERSITY OF MICHIGAN, NO. 70 THE LAND AND FRESH-WATER MOLLUSKS OF PUERTO RICO BY HENRY VAN DER SCHALIE ANN ARBOR UNIVERSITY OF MICHIGAN PRESS AUGUST12, 1948 MISCELLANEOUS PUBLICATIONS MUSEUM OF ZOOLOGY, UNIVERSITY OF MICHIGAN, NO. 70 THE LAND AND FRESH-WATER MOLLUSKS OF PUERTO RICO BY HENRY VAN DER SCHALIE ANN ARBOR UNIVERSITY OF MICHIGAN PRESS AUGUST12, 1948 CONTENTS INTRODUCTION.............. -... 9 Acknowledgments 10 ILLUSTRATIONS PLATES (Plates I-XITT follo~vpage 128) PLATE Francisco Mariaiio YagBn (front~spiece). I. FIG. 1. Alcadia striata (Lamnrck). FIG. a. Alcadia ILjulnlarsoni (Pfeiffer). FIG. 3. Alcadia ulta (Sowerby). FIa. 4. Helicina pl~asinnella " Sowel.by ' ' Pfeiff er. Fra. 5. Lucidella winosa (Sliuttle~vorth). PIG. 6. Lucidclla umbonuta (5huttlewortl1). FIa. 7. Pad?/enin portoricensis (Pfeiffer). FIG. 8. Ccrutoth.cc~isportoricanus Pilsbry and Vanatta. l17ra. 9. Stoaston~ops~U.C~~O~LC(LPLU $1. lj. 1l:lkcr. F1a. 10. Stoastonlops boriqucni 11. 13. Balter. 11. Fra. 1. Megalomastoma o'oceum (Ginelin). Fra. 2. Megalomasto?na werruculosum Sliuttlcworth. FIG. Licina decttssata (Lamarck). FIG. Licina aguadillensis (Pfeiffer) . FIG. Licina granainosa H. B. Baker. Fro. Chondropoma riisei (Pfeiffer). Fra. Chondropoma blauneri (Shuttleworth). Fra. Cl~ondropomaconseptum (von Martens). FIa. Chondropoma yunquei H. B. Baker. FIG. Chondroporna swifti (Sh~ttleworth). 111. FIG. 1. Pupi8,oma minus Pilsbry. FIG. 2. Pupison~adioscoricola (C. B. Adams). FIG. 3. Bothriopupa tenuidens (C. B. Adams). FIG. 4. Pupoides nitidulus (Pfeiffer) . FIG. 5. Gastrocopta sc.rwilis (Gould). FIG. 6. Gnstrocoptn prllncidn (Pfciffcr). Fra. 7. Guppya pi?~dlachi(Pf~iffer) . Fla. 8. Habroconcts cf. ernsti (Jousseaume). FIa. 9. Hau~aiia~tlinrrsc~rla (I%inney). -
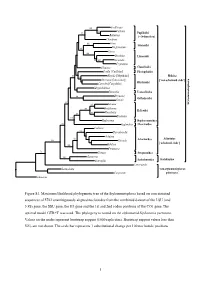
Figure S1. Maximum Likelihood Phylogenetic Tree of The
100 Cochlicopa 55 Vallonia 92 Pupilloidei Buliminus [= Orthurethra] Chondrina Arion 100 Arionoidei 66 Meghimatium Vitrina 100 Oxychilus Limacoidei 82 100 Euconulus Cryptozona Albinaria Clausilioidei Corilla [Corillidae] Plectopyloidea 70 Rhytida [Rhytididae] Helicina 53 Dorcasia [Dorcasiidae] [‘non-achatinoid clade’] Caryodes [Caryodidae] Rhytidoidei Megalobulimus Testacella Testacelloidea Drymaeus 94 Orthalicoidei Gaeotis 82 93 Satsuma Stylommatophora 100 Bradybaena Helicoidei Monadenia 87 93 84 Trochulus Haplotrema Haplotrematoidea 93 Euglandina Oleacinoidea Coeliaxis 92 Thyrophorella Achatina 92 Achatinina 100 Glessula Achatinoidea [‘achatinoid clade’] 100 Subulina Ferussacia 76 Gonaxis Streptaxoidea 100 Guestieria Systrophia Scolodontoidea Scolodontina Laevicaaulis Laemodonta ‘non-stylommatophoran Carychium pulmonates’ Siphonaria 1% 0.01 Figure S1. Maximum likelihood phylogenetic tree of the Stylommatophora based on concatenated sequences of 5782 unambiguously aligned nucleotides from the combined dataset of the LSU (and 5.8S) gene, the SSU gene, the H3 gene and the 1st and 2nd codon positions of the CO1 gene. The optimal model GTR+G was used. The phylogeny is rooted on the siphonariid Siphonaria pectinata. Values on the nodes represent bootstrap support (1000 replicates). Bootstrap support values less than 50% are not shown. The scale bar represents 1 substitutional change per 100 nucleotide positions. 1 91 Satsuma 100 Bradybaena Trochulus 97 Helicoidei 68 Monadenia 87 Haplotrema Haplotrematoidea Euglandina Oleacinoidea 100 Vallonia -

Subulina Octona (Bruguičre, 1798) – A
Malacologica Bohemoslovaca (2006), 5: 1–2 ISSN 1336-6939 Subulina octona (Bruguière, 1798) – a new greenhouse species for the Czech Republic (Mollusca: Gastropoda: Subulinidae) LUCIE JUŘIČKOVÁ Department of Zoology, Charles University, Viničná 7, CZ-12844 Praha 2, Czech Republic, e-mail: [email protected] JUŘIČKOVÁ L., 2006: Subulina octona (Bruguière, 1798) – a new greenhouse species for the Czech Re- public (Mollusca: Gastropoda: Subulinidae). – Malacologica Bohemoslovaca, 5: 1–2. Online serial at <http://mollusca.sav.sk> 30-Jan-2006. The occurrence of land snail Subulina octona (Bruguière 1798) (Gastropoda: Subulinidae) is reported from the Czech Republic greenhouse for the first time. Molluscan communities of two new Bohemian greenhouses are characterized. Introduction octona with flatter-sided, less tumid whorls and with Subulina octona (Bruguière 1798) is a tropical snail, much stronger and more regular growth-ridges, giving which naturally occurs in the Caribbean (DEISLER & a finely ribbed effect. Shell is also less glossy (Fig. 1). ABBOTT 1984) and in tropical America (PILSBRY Many reputed occurrences require confirmation, as 1946, DUNDEE 1974) where it is locally common. This both these species have several times been recorded in species has been introduced worldwide in the tropics error for other species of Subulinidae (KERNEY et al. (ABBOTT 1989, DEISLER & ABBOTT 1984, DUNDEE 1983, PILSBRY 1946). 1974, PILSBRY 1946). S. octona occurs in ground litter of moist places in tropical and subtropical forests, but it also occurs in open habitats (ALVAREZ & WILLIG 1993) and, in Nothern America and Europe (Britain, Ireland, Denmark and Germany), in greenhouses and hothouses. It feeds mostly on plant materials and de- bris. -

An Annotated List of the Non-Marine Mollusca of Britain and Ireland
JOURNAL OF CONCHOLOGY (2005), VOL.38, NO .6 607 AN ANNOTATED LIST OF THE NON-MARINE MOLLUSCA OF BRITAIN AND IRELAND ROY ANDERSON1 Abstract An updated nomenclatural list of the non-marine Mollusca of the Britain and Ireland is provided. This updates all previous lists and revises nomenclature and classification in the context of recent changes and of new European lists, including the Clecom List. Cases are made for the usage of names in the List by means of annotations. The List will provide a basis for the future census and cataloguing of the fauna of Britain and Ireland. Key words Taxonomic, list, nomenclature, non-marine, Mollusca, Britain, Ireland, annotated. INTRODUCTION There has been a need for some time to modernise the list of non-marine Mollusca for Britain and Ireland, a subject last visited in this journal in 1976 (Waldén 1976; Kerney 1976). Many of the changes that have appeared in the literature since then are contentious and Kerney (1999) chose not to incorporate many of these into the latest atlas of non-marine Mollusca of Britain and Ireland. A new European List, the Clecom List (Falkner et al. 2001) has now appeared and it seems appropriate to examine in more detail constituent changes which might affect the British and Irish faunas. This is given additional urgency by the inception of a new census of the molluscs of Britain and Ireland by the Conchological Society. Recorders in the Society are aware of many of the proposed changes but unable to implement them without general agreement. In addition, many field malacologists make use of the recording package RECORDER, a recent form of which has been developed jointly by JNCC and the National Biodiversity Network in the United Kingdom. -

Madagascar's Biogeographically Most Informative Land-Snail Taxa
Biogéographie de Madagascar,1996 :563-574 MADAGASCAR'S BIOGEOGRAPELICALLY MOST INFORMATIVE LAND- SNAIL TAXA Kenneth C. EMBERTON & Max F. RAKOTOMALALA Molluscar? Biodiversiiy Institute, 216-A Haddon Hills, Haddon$eld,NJ 08033, U.S.A. Departementd'Entomologie, Parc Botanique et Zoologiquede Tsimbazaza, Antananarivo 101, MADAGASCAR ABSTRACT.-Madagascar's known native land-snail faunais currently classifiedinto 540 species(97% endemic) in 68 genera (29% endemic)in 25 families (0% endemic). Recent survey work throughout the island may as much as double this number of species and should provide, for the first time, adequate material and distributional data for robust cladistic and biogeographic analyses. Preliminary analysisof existing cladograms and range maps suggestsareas of endenlism with recurrent patterns of vicariance. Which of the many Madagascan taxa will yieldthe most biogeographic information perunit of effort? Based on the criteria of species number, nzonophyly, vagility, character accessibility, and Gondwanan areas of endemism, the best candidates are (a) acavoids (giant, k-selected, (( bird's-egg snails D), (b) Boucardicus (minute, top-shaped shells with flamboyant apertures), (c) charopids (minute, discoid shells with complex microsculptures), and (d) streptaxids (small-to-medium-sized, white-shelled, high-spired carnivores). KEY-W0RDS.- Land-snail, Madagascar, Informative, Biogeography RESUME.- La faune connueà l'heure actuelle des escargots terrestres de Madagascar peut être classée dans 540 espèces (97% endémiques), 68 genres (29% endémiques) et 25 familles(0% endémiques). Un récent travail d'inventaire réalisé dans l'ensemble l'îlede pourra amener à doubler le nombre d'espèces et devra fournir pour la première fois un matériel et des données adéquates sur la distribution des espèces permettant des analyses cladistiques et biogéographiques robustes. -

Understanding the Remarkable Biodiversity of Príncipe Island – Scientific Report
www.fundacaoprincipe.org Understanding the Remarkable Biodiversity of Príncipe Island – Scientific Report Project Team: Frazer Sinclair, Yodiney Dos Santos, Ayres Pedronho, Aramis Andrade, Davide Dias, and Roldeney Fernandes. Report prepared by Frazer Sinclair, Fauna & Flora International, October 2019. For further information contact: [email protected], or [email protected] How to cite this document: Fundação Príncipe. (2019) Understanding the Remarkable Biodiversity of Príncipe Island – Scientific Report. Fundação Príncipe, Santo Antonio, Príncipe Island, www.fundacaoprincipe.org. Understanding the Remarkable Biodiversity of Príncipe Island – Scientific Report 1 www.fundacaoprincipe.org Summary Principe is home to a diversity of unique fauna and flora including globally threatened species such as the Critically Endangered Príncipe Thrush (Turdus xanthorhynchus) and the Vulnerable Obô Snail (Archachatina bicarinata). The implementation of conservation plans in Príncipe has previously been impeded by a lack of capacity and resources. Our project – Understanding the Remarkable Biodiversity of Prícipe Island – has built local capacity and implemented priority actions from existing conservation plans through the completion of a series of biological and social field surveys. These included an island wide survey of bird species using a point-count method; camera trap and nesting surveys to examine threats to the Príncipe Thrush; sampling of terrestrial molluscs at 10 sites across the island; & a questionnaire based survey of 14 communities to examine forest resource use and knowledge of the Obô Snail. Key findings included: An inventory of 32 bird species, with distribution models produced for 20 of these Confirmation of a March-July breeding period for the Príncipe thrush Camera trap images of non-native mammal species within the Parque Natural do Principe, including feral cats, dogs, African civets, Mona monkeys, and black rats. -

Gastropoda) of the Islands of Sao Tome and Principe, with New Records and Descriptions of New Taxa
This is the submitted version of the article: “Holyoak, D.T., Holyoak, G.A., Lima, R.F. de, Panisi, M. and Sinclair, F. 2020. A checklist of the land Mollusca (Gastropoda) of the islands of Sao Tome and Principe, with new records and descriptions of new taxa. Iberus, 38 (2): 219-319.” This version has not been peer-reviewed and is only being shared to comply with funder requirements. Please do not use it in any form and contact the authors (e.g.: [email protected]) to get access to the accepted version of the article. 1 New species and genera and new island records of land snails (Gastropoda) from the islands of São Tomé and Príncipe Nueva especies de .... David T. HOLYOAK1, Geraldine A. HOLYOAK1, Ricardo F. de LIMA2,3, Martina PANISI2 and Frazer SINCLAIR3,4 Recibidio el ... ABSTRACT Seven species of terrestrial Gastropoda are newly described from the island of São Tomé and six more from the island of Príncipe. The genera involved are Chondrocyclus (Cyclophoridae), Maizania and Thomeomaizania (Maizaniidae), Pseudoveronicella (Veronicellidae), Nothapalus (Achatinidae: subfamily undet.), Gulella and Streptostele (Streptaxidae), Truncatellina (Truncatellinidae), Afroconulus (Euconulidae), Principicochlea gen. nov., Principotrochoidea gen. nov., Thomithapsia gen. nov. and Thomitrochoidea gen. nov. (Urocyclidae). Most of these are from natural forest habitats and are likely to be single- island endemics. Apothapsia gen. nov. (Helicarionidae) is also described to accommodate two previously known species. Additional new island records are of ten species on São Tomé, one on Príncipe alone and two more on both islands. These include six species of "microgastropods" with wider ranges in tropical Africa that are likely to be hitherto overlooked parts of the indigenous fauna and six anthropogenic introductions; Pseudopeas crossei previously known only from Príncipe and Bioko is newly recorded on São Tomé. -

How to Cite Complete Issue More Information About This Article
Acta zoológica mexicana ISSN: 0065-1737 ISSN: 2448-8445 Instituto de Ecología A.C. Castillo-Rodríguez, Zoila G.; Naranjo-García, Edna; Amezcua-Linares, Felipe A new record of Huttonella bicolor (Hutton, 1834) (Mollusca, Gastropoda, Streptaxidae) in Mexico Acta zoológica mexicana, vol. 34, e3411181, 2018 Instituto de Ecología A.C. DOI: 10.21829/azm.2018.3411181 Available in: http://www.redalyc.org/articulo.oa?id=57560238005 How to cite Complete issue Scientific Information System Redalyc More information about this article Network of Scientific Journals from Latin America and the Caribbean, Spain and Journal's webpage in redalyc.org Portugal Project academic non-profit, developed under the open access initiative e ISSN 2448-8445 (2018) Volumen34, 1–6 ISSN 0065-1737 elocation- id: e3411181 DOI: https://doi.org/10.21829/azm.2018.3411181 Artículo original (Original paper) A NEW RECORD OF HUTTONELLA BICOLOR (HUTTON, 1834) (MOLLUSCA, GASTROPODA, STREPTAXIDAE) IN MEXICO UN NUEVO REGISTRO DE HUTTONELLA BICOLOR (MOLLUSCA, GASTROPODA, STREPTAXIDAE) EN MEXICO Zoila G. CASTILLO-RODRÍGUEZ,1,* Edna NARANJO-GARCÍA,2 and Felipe AMEZCUA-LINARES1 1Instituto de Ciencias del Mar y Limnología, Departamento de Biodiversidad y Ecología Acuática, Universidad Nacional Autónoma de México, Apartado Postal 70-305, Cd. de México. C.P. 04510. <[email protected]>; <[email protected]> 2Instituto de Biología, Departamento de Zoología, Universidad Nacional Autónoma de México, Apartado Postal 70-153, Cd. de México C.P. 04510. <[email protected]> *Autor para correspondencia: <[email protected]> Recibido: 13/06/2017; aceptado: 20/10/2017; publicado en línea: 16/03/2018 Editor responsable: Alfonso Correa Sandoval Castillo-Rodríguez, Z. -
![List of Regulated Living Organisms Under the Invasive Alien Species Act [Animal Kingdom]](https://docslib.b-cdn.net/cover/0745/list-of-regulated-living-organisms-under-the-invasive-alien-species-act-animal-kingdom-3670745.webp)
List of Regulated Living Organisms Under the Invasive Alien Species Act [Animal Kingdom]
List of Regulated Living Organisms under the Invasive Alien Species Act [Animal Kingdom] Designated on June 1, 2005 Designated on Oct 1, 2015 Designated on February 1, 2006 Designated on Oct 1, 2016 Designated on September 1, 2006 Designated on Jan 15, 2018 Designated on September 1, 2007 Designated on Apr 1, 2018 Designated on January 1, 2008 Designated on Nov.2, 2020 Designated on February 1, 2010 Designated on July 1, 2011 Designated on September 1, 2013 Designated on June 11, 2014 Designated on Aug 1, 2014 Designated on Mar 1, 2015 Living Organisms Required to have a Certificate Attached during their Invasive Alien Species Unevaluated Alien Species Class Order Family Genus importation in order to verify their (IAS) (UAS) types (LORCA) Didelphis None Any species of the genus Didelphis Didelphidae All other genera of None None Didelphidae Any species of the families Marsupialia Brushtail possum Any species of the family Trichosurus Didelphidae and Phalangeridae (T. vulpecula ) Phalangeridae excluding spotted Phalangeridae All other genera of cuscus (Spilocuscus maculatus ) and None Phalangeridae brushtail possum (T. vulpecula ) Any species of the genus Erinaceus None Erinaceus Any species of the genera Insectivora Erinaceidae Atelerix Any species of the genera Atelerix , Erinaceus , Atelerix , Hemiechinus , Hemiechinus None Hemiechinus and Mesechinus and Mesechinus Mesechinus excluding Atelerix albiventris Taiwan macaque Any species of the genus Macaca (M. cyclopis ) excluding Japanese macaque (M. fuscata) ,Taiwan macaque (M. Crab-eating macaque Any species of the genus Macaca (M. fascicularis ) cyclopis) , crab-eating macaque (M. Rhesus macaque fascicularis) , and rhesus macaque (M. mulatta ) (M. mulatta) Primates Cercopithecidae Macaca Taiwan macaque × Japanese Any living hybrid organisms of macaque species of the genus Macaca excluding Taiwan macaque × ( M.