Vocal Communication in a Tolerant Multi-Level Society: Insights from Signallers and Receivers in Guinea Baboons
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Open Source Subtitle Editor Software Study for Section 508 Close Caption Applications
https://ntrs.nasa.gov/search.jsp?R=20140002618 2019-08-29T14:44:57+00:00Z Open Source Subtitle Editor Software Study for Section 508 Close Caption Applications Brandon F. Murphy NASA. John F. Kennedy Space Center Major: Computer Science USRP Summer 2013 Session Date: July 30 2013 NASA USRP - Internship Final Report Open Source Subtitle Editor Software Study for Section 508 Close Caption Applications Brandon F. Murphy North Carolina Agricultural and Technical State University Greensboro, North Carolina. 27411 Nomenclature cc = Closed Captioning !TAW = IT Accessibility and Workforce GSA = General Services Administration EfT = Electronic and Information Technology .srt = the file extension for Sub Rip subtitle files oss = Open Source Software .ssa = SubStation Alpha .ass = Advanced SubStation Alpha Subtitle .sub = Subtitling file .txt = Text File .idx = Index file I. Introduction Government agencies are required by law under the Section 508 policy to provide any tools or services for their employees who may need them. For those who need special assistance these tools and services help them do their job to the best of their ability. With these tools and services a person with disabilities will have equal opportunities for advancing in their company, as others do. This research and implementation was used to find cost effective solutions to make information available to all Government employees as required by law, Section 508. II. Abstract This paper will focus on a specific item within the NASA Electronic Information Accessibility Policy - Multimedia Presentation shall have synchronized caption; thus making information accessible to a person with hearing impairment. This synchronized caption will assist a person with hearing or cognitive disability to access the same information as everyone else. -

Virtualdub Tutorial
Using VirtualDub to edit and recompress videos made by cameras like MD-80 or 808 KeyChain Live long and prosper. I do not pretend to teach anything to anyone, but I hope to give some suggestions to help those unfamiliar with the program. My scope is to help people who want to change (e.g. duration or remove timestamp) and then recompress videos, such as DivX. The videos produced by the cameras are usually compressed in MPEG-4 (standard DVD) and are very large. Here are the steps to follow. I’ve written which are mandatory and which optional. INSTALLING VirtualDub (required) Go to this webpage and download VirtualDub: http://virtualdub.sourceforge.net/ Search the first link: List download mirrors for Vx.xx (32-bit) release build. It’s not a program to be installed. Simply unzip the downloaded archive in the folder you want. I've put it here: C:\Program Files\VirtualDub and I made a link to VirtualDub.exe on desktop for convenience. DOWNLOAD PLUG-IN TO REMOVE TIMESTAMP (optional) Open this webpage: http://www.aircommandrockets.com/md80clone.htm and download the file: timestamp.vdf (Thanks to George) Once downloaded, copy it here: C:\Program Files\VirtualDub\plugins (where C:\Program Files\VirtualDub\ is the path where you extracted the archive above) An alternate filter to remove the timestamp is called Logoaway and can be found here: http://voidon.republika.pl/virtualdub/ (Thanks to Krzysztof Wojdon) DOWNLOAD DivX CODEC (optional if already installed) Open this page: http://www.divx.com/en/software/divx-plus and press the Free Download button (green). -

This Electronic Thesis Or Dissertation Has Been Downloaded from Explore Bristol Research
This electronic thesis or dissertation has been downloaded from Explore Bristol Research, http://research-information.bristol.ac.uk Author: Gilmour, Lia R V Title: Evaluating methods to deter bats General rights Access to the thesis is subject to the Creative Commons Attribution - NonCommercial-No Derivatives 4.0 International Public License. A copy of this may be found at https://creativecommons.org/licenses/by-nc-nd/4.0/legalcode This license sets out your rights and the restrictions that apply to your access to the thesis so it is important you read this before proceeding. Take down policy Some pages of this thesis may have been removed for copyright restrictions prior to having it been deposited in Explore Bristol Research. However, if you have discovered material within the thesis that you consider to be unlawful e.g. breaches of copyright (either yours or that of a third party) or any other law, including but not limited to those relating to patent, trademark, confidentiality, data protection, obscenity, defamation, libel, then please contact [email protected] and include the following information in your message: •Your contact details •Bibliographic details for the item, including a URL •An outline nature of the complaint Your claim will be investigated and, where appropriate, the item in question will be removed from public view as soon as possible. Evaluating methods to deter bats Lia Rose Vincent Gilmour A dissertation submitted to the University of Bristol in accordance with the requirements for award of the degree of PhD in the Faculty of Life Sciences. School of Biological Sciences, University of Bristol October 2019 34,189 words Abstract Bat deterrents, such as radar and acoustic methods have been suggested as potential mitigation for when bats are at risk of harm from human activities and/or structures. -

Western Gorilla Social Structure and Inter-Group Dynamics
Western Gorilla Social Structure and Inter-Group Dynamics Robin Morrison Darwin College This dissertation is submitted for the degree of Doctor of Philosophy March 2019 Declaration of Originality This dissertation is the result of my own work and includes nothing which is the outcome of work done in collaboration except as declared in the Preface and specified in the text. It is not substantially the same as any that I have submitted, or, is being concurrently submitted for a degree or diploma or other qualification at the University of Cambridge or any other University or similar institution except as declared in the Preface and specified in the text. I further state that no substantial part of my dissertation has already been submitted, or, is being concurrently submitted for any such degree, diploma or other qualification at the University of Cambridge or any other University or similar institution except as declared in the Preface and specified in the text. Statement of Length The word count of this dissertation is 44,718 words excluding appendices and references. It does not exceed the prescribed word limit for the Archaeology and Anthropology Degree Committee. II Western Gorilla Social Structure and Inter-Group Dynamics Robin Morrison The study of western gorilla social behaviour has primarily focused on family groups, with research on inter-group interactions usually limited to the interactions of a small number of habituated groups or those taking place in a single location. Key reasons for this are the high investment of time and money required to habituate and monitor many groups simultaneously, and the difficulties of making observations on inter-group social interaction in dense tropical rainforest. -

Linux Mint - 2Nde Partie
Linux Mint - 2nde partie - Mise à jour du 10.03.2017 1 Sommaire 1. Si vous avez raté l’épisode précédent… 2. Utiliser Linux Mint au quotidien a) Présentation de la suite logicielle par défaut b) Et si nous testions un peu ? c) Windows et Linux : d’une pratique logicielle à une autre d) L’installation de logiciels sous Linux 3. Vous n’êtes toujours pas convaincu(e)s par Linux ? a) Encore un argument : son prix ! b) L’installer sur une vieille ou une nouvelle machine, petite ou grande c) Par philosophie et/ou curiosité d) Pour apprendre l'informatique 4. À retenir Sources 2 1. Si vous avez raté l’épisode précédent… Linux, c’est quoi ? > Un système d’exploitation > Les principaux systèmes d'exploitation > Les distributions 3 1. Si vous avez raté l’épisode précédent… Premiers pas avec Linux Mint > Répertoire, dossier ou fichier ? > Le bureau > Gestion des fenêtres > Gestion des fichiers 4 1. Si vous avez raté l’épisode précédent… Installation > Méthode « je goûte ! » : le LiveUSB > Méthode « j’essaye ! » : le dual-boot > Méthode « je fonce ! » : l’installation complète 5 1. Si vous avez raté l’épisode précédent… Installation L'abréviation LTS signifie Long Term Support, ou support à long terme. 6 1. Si vous avez raté l’épisode précédent… http://www.linuxliveusb.com 7 1. Si vous avez raté l’épisode précédent… Installation 8 1. Si vous avez raté l’épisode précédent… Installation 9 1. Si vous avez raté l’épisode précédent… Installation 10 1. Si vous avez raté l’épisode précédent… Installation 11 2. Utiliser Linux Mint au quotidien a) Présentation de la suite logicielle par défaut Le fichier ISO Linux Mint est compressé et contient environ 1,6 GB de données. -

10 Best Free Video Editing Software Review and Download
Copy Right www.imelfin.com 10 best free video editing software review and download What are the best free video editing software? In this post, you are bound to find the best video editing freeware to your taste. When we want to create a personal video, burn a DVD, or upload to YouTube/other video-sharing sites, we will need free video editing software to crop, edit subtitles, insert audio, and add other effects. So what is the best free video editing software? What is the easiest video editing software to use? This post covers a list of the best video editing software, you will be able to edit your videos free on Windows, Mac or Linux. 10 best free video editing software review and download 1. ezvid free video editing software 1 Copy Right www.imelfin.com ezvid is open-source video editing software applicable to Windows XP (SP3), Vista, Win 7/8. It is fast, easy-to-use and functional. Besides video editing, it can also be used as a voice recorder and screen recorder. As with video editing, ezvid enables you to resize, add text/images. Powerful functions along with the revolutionary screen drawing feature have made ezvid one of the best video editing freeware. ezvid video editing software free download 2. Windows Movie Maker 2 Copy Right www.imelfin.com Windows Movie Maker offers a simple solution to beginners to create or edit videos. You can drag and drop images, videos as well as the real-time screenshots/videos to the timeline and add titles, credits, video/transition effects as you like. -
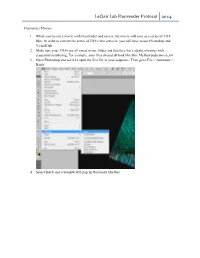
Leclair Lab Fluorender Protocol 2014
LeClair Lab Fluorender Protocol 2014 Fluorender Movies 1. When you record a movie with Fluorender and save it, the movie will save as a series of TIFF files. In order to convert the series of TIFFs into a movie, you will have to use Photoshop and VirtualDub. 2. Make sure your TIFFs are all saved in one folder and that they have identical names with sequential numbering. For example, your files should all look like this: Myfluorendermovie_00 3. Open Photoshop and use it to open the first file in your sequence. Then go to File > Automate > Batch. 4. Select Batch and a window will pop up that looks like this. LeClair Lab Fluorender Protocol 2014 5. Under Play, select Default Actions form the dropdown menu. In the second dropdown menu, select File Format. This will convert your TIFF files to JPEG files. If you would like another file format, you can make your own action fairly easily. a. To make your own action, go back to the editing window and press Alt + F9. The actions window will pop up. In the dropdown action menu, select new action. This will begin “recording” your actions, so after this point it is important that you do not click anything other than what you want to make into an action. LeClair Lab Fluorender Protocol 2014 b. Now, perform the task that you would like to be completed in each image. For example, if you want to perform a file conversion go to File > Save As and select the Format in which you would like your file to be saved. -

Social Strategies and Tool Use in Wild Corvids
Social strategies and tool use in wild corvids Thèse présentée à la Faculté des Sciences Institut de Biologie, Université de Neuchâtel Pour l’obtention du grade de docteur ès sciences Par Di Lascio Felice Elias Acceptée sur proposition du jury : Prof. Redouan Bshary, directeur de thèse Prof. Thomas Bugnyar, rapporteur Prof. Klaus Zuberbühler, rapporteur Présentée et soutenue le 28. 01. 2014 1 2 Faculté des sciences Secrétariat-décanat de Faculté Rue Emile-Argand 11 2000 Neuchâtel - Suisse Tél: + 41 (0)32 718 2100 E-mail: [email protected] IMPRIMATUR POUR THESE DE DOCTORAT La Faculté des sciences de l'Université de Neuchâtel autorise l'impression de la présente thèse soutenue par Monsieur Felice Di Lascio Titre: “Social strategies and tool use in wild corvids” sur le rapport des membres du jury composé comme suit: - Prof. Redouan Bshary, Université de Neuchâtel, directeur de thèse - Prof. Klaus Zuberbühler, Université de Neuchâtel - Prof. Thomas Bugnyar, Université de Vienne, Autriche Neuchâtel, 25 février 2014 Le Doyen, Prof. P. Kropf 3 Imprimatur pour thèse de doctorat www.unine.ch/sciences 4 Content (1/2) Content Pages Imprimatur 3 Summary (English) 9-10 Résumé (French) 11-13 General introduction 15-29 Chapter 1 (First manuscript: Pair partners in rooks (Corvus frugilegus) coordinate to cope with the costs of theft of nest material) (a) Abstract 31-32 (b) Introduction 32-35 (c) Methods 35-38 (d) Results 39-42 (e) Discussion 43-45 (f) Acknowledgments 45 (g) References 45-48 5 6 Content (2/2) Content Pages Chapter 2 (Second -

Learning Virtualdub the Complete Guide to Capturing, Processing, and Encoding Digital Video
Learning VirtualDub The complete guide to capturing, processing, and encoding digital video Georgios Diamantopoulos Sohail Salehi John Buechler Birmingham - Mumbai Learning VirtualDub The complete guide to capturing, processing, and encoding digital video Copyright © 2005 Packt Publishing All rights reserved. No part of this book may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, without the prior written permission of the publisher, except in the case of brief quotations embedded in critical articles or reviews. Every effort has been made in the preparation of this book to ensure the accuracy of the information presented. However, the information contained in this book is sold without warranty, either express or implied. Neither the authors, Packt Publishing, nor its dealers or distributors will be held liable for any damages caused or alleged to be caused either directly or indirectly by this book. Packt Publishing has endeavored to provide trademark information about all the companies and products mentioned in this book by the appropriate use of capitals. However, Packt Publishing cannot guarantee the accuracy of this information. First edition: April 2005 Published by Packt Publishing Ltd. 32 Lincoln Road Olton Birmingham, B27 6PA, UK. ISBN 1-904811-35-3 www.packtpub.com Cover Design by www.visionwt.com Credits Authors Layout Georgios Diamantopoulos Paramita Chakrabarti Sohail Salehi John Buechler Indexer Ashutosh Pande Commissioning Editor David Barnes Proofreader Chris Smith Technical Editors Ashutosh Pande Cover Designer Paramita Chakrabarti Helen Wood About the Authors Georgios Diamantopoulos was born in February 1984. Born and raised in Corinth, Greece, his scientific inclination towards computers was evident in his teenage years and he pursued his dreams by moving to Birmingham, UK to study Computer Systems Engineering in 2001. -

Multimedia Good,Better,Best
MULTIMEDIA GOOD, BETTER, BEST This document provides good / better / best options for audio and moving image digitization workstations. While the equipment listed is geared to deal with media on magnetic tape (e.g., audio cassettes and VHS), we briefly discuss some options for digitizing film. The products currently used by Washington State University Libraries are highlighted as an example of a suite of options. The devices we mention provide an idea of what is available in different price ranges. Generally speaking, the products in the better categories provide more options than those in the good, and those in the best provide more options than those in the better. While using the best equipment gives an institution the greatest opportunity to digitize a wide range of media at a desirable level of quality, there are many situations where extra options are not necessary for the sort of work that takes place at most archival institutions. It is also very important to define the needs of an institution before purchasing any piece of equipment. This includes defining the specifications for digital files and determining the amount of materials that need to be digitized. For one-off projects, it is often wise to consider hiring a reputable third-party contractor to perform the digitization and at least some of the metadata creation. Most any audiovisual digitization workflow will involve a playback device to read the original analog medium, a device to convert the analog signal to digital, software to create the digital files that the institution will curate, and a computer that will run the software. -
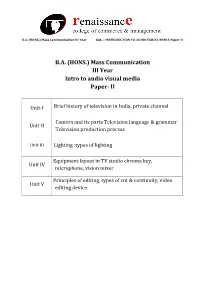
Mass Communication III Year Intro to Audio Visual Media Paper- II
B.A. (HONS.) Mass Communication III Year Sub. – INTRODUCTION TO AUDIO VISUAL MEDIA Paper II B.A. (HONS.) Mass Communication III Year Intro to audio visual media Paper- II Unit-I Brief history of television in India, private channel Camera and its parts Television language & grammar Unit-II Television production process Unit III Lighting :types of lighting Equipment layout in TV studio chroma key, Unit IV microphone, vision mixer Principles of editing, types of cut & continuity, video Unit V editing device B.A. (HONS.) Mass Communication III Year Sub. – INTRODUCTION TO AUDIO VISUAL MEDIA Paper II Unit-I Brief history of television in India, private channel Television came to India on September 15, 1959 with experimental transmission from Delhi. It was a modest beginning with a make shift studio, a low power transmitter and only 21 community television sets.All India Radio provided the engineering and programme professionals. A daily one-hour service with a news bulletin was started in 1965. In1972 television services were extended to a second city—Mumbai. By1975 television stations came up in Calcutta, Chennai, Srinagar, Amritsar and Lucknow. In 1975-76 the Satellite Instructional Television Experiment brought television programmes for people in 2400 villages inthe most inaccessible of the least developed areas tlirough a satellite lentto India for one year.Doordarshan is a Public broadcast terrestrial ltelevision channel run by Prasar Bharati, a board formedby the Government of India. It is one of the largest broadcasting organizations in the world in terms of the of studios and transmitters. Doordarshanhad its beginning with the experimental telecast started in Delhi in September, 1959 with a small transmitter and a makeshift studio. -

Animal Abuse As a Sentinel for Human Violence: a Critique ∗ Emily G
Journal of Social Issues, Vol. 65, No. 3, 2009, pp. 589--614 Animal Abuse as a Sentinel for Human Violence: A Critique ∗ Emily G. Patterson-Kane American Veterinary Medical Association Heather Piper Manchester Metropolitan University It has been suggested that acts of violence against human and nonhuman an- imals share commonalities, and that animal abuse is a sentinel for current or future violence toward people. The popular and professional acceptance of strong connections between types of violence is beginning to be used to justify social work interventions and to influence legal decision making, and so requires greater scrutiny. Examination of the limited pool of empirical data suggests that animal abuse is relatively common among men, with violent offenders having an increased probability of reporting prior animal abuse—with the majority of violent offend- ers not reporting any animal abuse. Causal explanations for “the link,” such as empathy impairment or conduct disorder, suffer from a lack of validating research and, based on research into interhuman violence, the assumption that violence has a predominant, single underlying cause must be questioned. An (over)emphasis on the danger that animal abusers pose to humans serves to assist in achieving a consensus that animal abuse is a serious issue, but potentially at the cost of failing to focus on the most common types of abuse, and the most effective strategies for reducing its occurrence. Nothing in this review and discussion should be taken as minimizing the importance of animals as frequent victims of violence, or the co-occurrence of abuse types in “at-risk” households.