This Electronic Thesis Or Dissertation Has Been Downloaded from Explore Bristol Research
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Open Source Subtitle Editor Software Study for Section 508 Close Caption Applications
https://ntrs.nasa.gov/search.jsp?R=20140002618 2019-08-29T14:44:57+00:00Z Open Source Subtitle Editor Software Study for Section 508 Close Caption Applications Brandon F. Murphy NASA. John F. Kennedy Space Center Major: Computer Science USRP Summer 2013 Session Date: July 30 2013 NASA USRP - Internship Final Report Open Source Subtitle Editor Software Study for Section 508 Close Caption Applications Brandon F. Murphy North Carolina Agricultural and Technical State University Greensboro, North Carolina. 27411 Nomenclature cc = Closed Captioning !TAW = IT Accessibility and Workforce GSA = General Services Administration EfT = Electronic and Information Technology .srt = the file extension for Sub Rip subtitle files oss = Open Source Software .ssa = SubStation Alpha .ass = Advanced SubStation Alpha Subtitle .sub = Subtitling file .txt = Text File .idx = Index file I. Introduction Government agencies are required by law under the Section 508 policy to provide any tools or services for their employees who may need them. For those who need special assistance these tools and services help them do their job to the best of their ability. With these tools and services a person with disabilities will have equal opportunities for advancing in their company, as others do. This research and implementation was used to find cost effective solutions to make information available to all Government employees as required by law, Section 508. II. Abstract This paper will focus on a specific item within the NASA Electronic Information Accessibility Policy - Multimedia Presentation shall have synchronized caption; thus making information accessible to a person with hearing impairment. This synchronized caption will assist a person with hearing or cognitive disability to access the same information as everyone else. -

Virtualdub Tutorial
Using VirtualDub to edit and recompress videos made by cameras like MD-80 or 808 KeyChain Live long and prosper. I do not pretend to teach anything to anyone, but I hope to give some suggestions to help those unfamiliar with the program. My scope is to help people who want to change (e.g. duration or remove timestamp) and then recompress videos, such as DivX. The videos produced by the cameras are usually compressed in MPEG-4 (standard DVD) and are very large. Here are the steps to follow. I’ve written which are mandatory and which optional. INSTALLING VirtualDub (required) Go to this webpage and download VirtualDub: http://virtualdub.sourceforge.net/ Search the first link: List download mirrors for Vx.xx (32-bit) release build. It’s not a program to be installed. Simply unzip the downloaded archive in the folder you want. I've put it here: C:\Program Files\VirtualDub and I made a link to VirtualDub.exe on desktop for convenience. DOWNLOAD PLUG-IN TO REMOVE TIMESTAMP (optional) Open this webpage: http://www.aircommandrockets.com/md80clone.htm and download the file: timestamp.vdf (Thanks to George) Once downloaded, copy it here: C:\Program Files\VirtualDub\plugins (where C:\Program Files\VirtualDub\ is the path where you extracted the archive above) An alternate filter to remove the timestamp is called Logoaway and can be found here: http://voidon.republika.pl/virtualdub/ (Thanks to Krzysztof Wojdon) DOWNLOAD DivX CODEC (optional if already installed) Open this page: http://www.divx.com/en/software/divx-plus and press the Free Download button (green). -

Linux Mint - 2Nde Partie
Linux Mint - 2nde partie - Mise à jour du 10.03.2017 1 Sommaire 1. Si vous avez raté l’épisode précédent… 2. Utiliser Linux Mint au quotidien a) Présentation de la suite logicielle par défaut b) Et si nous testions un peu ? c) Windows et Linux : d’une pratique logicielle à une autre d) L’installation de logiciels sous Linux 3. Vous n’êtes toujours pas convaincu(e)s par Linux ? a) Encore un argument : son prix ! b) L’installer sur une vieille ou une nouvelle machine, petite ou grande c) Par philosophie et/ou curiosité d) Pour apprendre l'informatique 4. À retenir Sources 2 1. Si vous avez raté l’épisode précédent… Linux, c’est quoi ? > Un système d’exploitation > Les principaux systèmes d'exploitation > Les distributions 3 1. Si vous avez raté l’épisode précédent… Premiers pas avec Linux Mint > Répertoire, dossier ou fichier ? > Le bureau > Gestion des fenêtres > Gestion des fichiers 4 1. Si vous avez raté l’épisode précédent… Installation > Méthode « je goûte ! » : le LiveUSB > Méthode « j’essaye ! » : le dual-boot > Méthode « je fonce ! » : l’installation complète 5 1. Si vous avez raté l’épisode précédent… Installation L'abréviation LTS signifie Long Term Support, ou support à long terme. 6 1. Si vous avez raté l’épisode précédent… http://www.linuxliveusb.com 7 1. Si vous avez raté l’épisode précédent… Installation 8 1. Si vous avez raté l’épisode précédent… Installation 9 1. Si vous avez raté l’épisode précédent… Installation 10 1. Si vous avez raté l’épisode précédent… Installation 11 2. Utiliser Linux Mint au quotidien a) Présentation de la suite logicielle par défaut Le fichier ISO Linux Mint est compressé et contient environ 1,6 GB de données. -

10 Best Free Video Editing Software Review and Download
Copy Right www.imelfin.com 10 best free video editing software review and download What are the best free video editing software? In this post, you are bound to find the best video editing freeware to your taste. When we want to create a personal video, burn a DVD, or upload to YouTube/other video-sharing sites, we will need free video editing software to crop, edit subtitles, insert audio, and add other effects. So what is the best free video editing software? What is the easiest video editing software to use? This post covers a list of the best video editing software, you will be able to edit your videos free on Windows, Mac or Linux. 10 best free video editing software review and download 1. ezvid free video editing software 1 Copy Right www.imelfin.com ezvid is open-source video editing software applicable to Windows XP (SP3), Vista, Win 7/8. It is fast, easy-to-use and functional. Besides video editing, it can also be used as a voice recorder and screen recorder. As with video editing, ezvid enables you to resize, add text/images. Powerful functions along with the revolutionary screen drawing feature have made ezvid one of the best video editing freeware. ezvid video editing software free download 2. Windows Movie Maker 2 Copy Right www.imelfin.com Windows Movie Maker offers a simple solution to beginners to create or edit videos. You can drag and drop images, videos as well as the real-time screenshots/videos to the timeline and add titles, credits, video/transition effects as you like. -
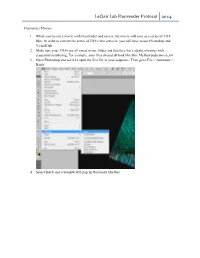
Leclair Lab Fluorender Protocol 2014
LeClair Lab Fluorender Protocol 2014 Fluorender Movies 1. When you record a movie with Fluorender and save it, the movie will save as a series of TIFF files. In order to convert the series of TIFFs into a movie, you will have to use Photoshop and VirtualDub. 2. Make sure your TIFFs are all saved in one folder and that they have identical names with sequential numbering. For example, your files should all look like this: Myfluorendermovie_00 3. Open Photoshop and use it to open the first file in your sequence. Then go to File > Automate > Batch. 4. Select Batch and a window will pop up that looks like this. LeClair Lab Fluorender Protocol 2014 5. Under Play, select Default Actions form the dropdown menu. In the second dropdown menu, select File Format. This will convert your TIFF files to JPEG files. If you would like another file format, you can make your own action fairly easily. a. To make your own action, go back to the editing window and press Alt + F9. The actions window will pop up. In the dropdown action menu, select new action. This will begin “recording” your actions, so after this point it is important that you do not click anything other than what you want to make into an action. LeClair Lab Fluorender Protocol 2014 b. Now, perform the task that you would like to be completed in each image. For example, if you want to perform a file conversion go to File > Save As and select the Format in which you would like your file to be saved. -

Learning Virtualdub the Complete Guide to Capturing, Processing, and Encoding Digital Video
Learning VirtualDub The complete guide to capturing, processing, and encoding digital video Georgios Diamantopoulos Sohail Salehi John Buechler Birmingham - Mumbai Learning VirtualDub The complete guide to capturing, processing, and encoding digital video Copyright © 2005 Packt Publishing All rights reserved. No part of this book may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, without the prior written permission of the publisher, except in the case of brief quotations embedded in critical articles or reviews. Every effort has been made in the preparation of this book to ensure the accuracy of the information presented. However, the information contained in this book is sold without warranty, either express or implied. Neither the authors, Packt Publishing, nor its dealers or distributors will be held liable for any damages caused or alleged to be caused either directly or indirectly by this book. Packt Publishing has endeavored to provide trademark information about all the companies and products mentioned in this book by the appropriate use of capitals. However, Packt Publishing cannot guarantee the accuracy of this information. First edition: April 2005 Published by Packt Publishing Ltd. 32 Lincoln Road Olton Birmingham, B27 6PA, UK. ISBN 1-904811-35-3 www.packtpub.com Cover Design by www.visionwt.com Credits Authors Layout Georgios Diamantopoulos Paramita Chakrabarti Sohail Salehi John Buechler Indexer Ashutosh Pande Commissioning Editor David Barnes Proofreader Chris Smith Technical Editors Ashutosh Pande Cover Designer Paramita Chakrabarti Helen Wood About the Authors Georgios Diamantopoulos was born in February 1984. Born and raised in Corinth, Greece, his scientific inclination towards computers was evident in his teenage years and he pursued his dreams by moving to Birmingham, UK to study Computer Systems Engineering in 2001. -

Multimedia Good,Better,Best
MULTIMEDIA GOOD, BETTER, BEST This document provides good / better / best options for audio and moving image digitization workstations. While the equipment listed is geared to deal with media on magnetic tape (e.g., audio cassettes and VHS), we briefly discuss some options for digitizing film. The products currently used by Washington State University Libraries are highlighted as an example of a suite of options. The devices we mention provide an idea of what is available in different price ranges. Generally speaking, the products in the better categories provide more options than those in the good, and those in the best provide more options than those in the better. While using the best equipment gives an institution the greatest opportunity to digitize a wide range of media at a desirable level of quality, there are many situations where extra options are not necessary for the sort of work that takes place at most archival institutions. It is also very important to define the needs of an institution before purchasing any piece of equipment. This includes defining the specifications for digital files and determining the amount of materials that need to be digitized. For one-off projects, it is often wise to consider hiring a reputable third-party contractor to perform the digitization and at least some of the metadata creation. Most any audiovisual digitization workflow will involve a playback device to read the original analog medium, a device to convert the analog signal to digital, software to create the digital files that the institution will curate, and a computer that will run the software. -
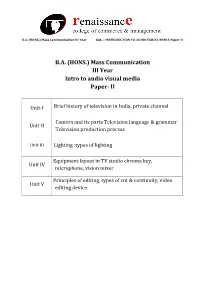
Mass Communication III Year Intro to Audio Visual Media Paper- II
B.A. (HONS.) Mass Communication III Year Sub. – INTRODUCTION TO AUDIO VISUAL MEDIA Paper II B.A. (HONS.) Mass Communication III Year Intro to audio visual media Paper- II Unit-I Brief history of television in India, private channel Camera and its parts Television language & grammar Unit-II Television production process Unit III Lighting :types of lighting Equipment layout in TV studio chroma key, Unit IV microphone, vision mixer Principles of editing, types of cut & continuity, video Unit V editing device B.A. (HONS.) Mass Communication III Year Sub. – INTRODUCTION TO AUDIO VISUAL MEDIA Paper II Unit-I Brief history of television in India, private channel Television came to India on September 15, 1959 with experimental transmission from Delhi. It was a modest beginning with a make shift studio, a low power transmitter and only 21 community television sets.All India Radio provided the engineering and programme professionals. A daily one-hour service with a news bulletin was started in 1965. In1972 television services were extended to a second city—Mumbai. By1975 television stations came up in Calcutta, Chennai, Srinagar, Amritsar and Lucknow. In 1975-76 the Satellite Instructional Television Experiment brought television programmes for people in 2400 villages inthe most inaccessible of the least developed areas tlirough a satellite lentto India for one year.Doordarshan is a Public broadcast terrestrial ltelevision channel run by Prasar Bharati, a board formedby the Government of India. It is one of the largest broadcasting organizations in the world in terms of the of studios and transmitters. Doordarshanhad its beginning with the experimental telecast started in Delhi in September, 1959 with a small transmitter and a makeshift studio. -

Video Analysis of Head Kinematics in Boxing Matches Using Opencv Library Under Macintosh Platform How Can the Posit Algorithm Be Used in Head Kinematic Analysis?
IT 12 046 Examensarbete 30 hp September 2012 Video analysis of head kinematics in boxing matches using OpenCV library under Macintosh platform How can the Posit algorithm be used in head kinematic analysis? Liyi Zhao Institutionen för informationsteknologi Department of Information Technology Abstract Video analysis of head kinematics in boxing matches using OpenCV library under Macintosh platform Liyi Zhao Teknisk- naturvetenskaplig fakultet UTH-enheten The division of Neuronic Engineering at KTH focuses the research on the head and neck biomechanics. Besöksadress: Finite Element (FE) models of the human neck and Ångströmlaboratoriet Lägerhyddsvägen 1 head have been developed to study the neck and head Hus 4, Plan 0 kinematics as well as injurious loadings of various kinds. The overall objective is to improve the injury Postadress: prediction through accident reconstruction. Box 536 751 21 Uppsala This project aims at providing an image analysis tool Telefon: which helps analyzers building models of the head 018 – 471 30 03 motion, making good estimation of head movements, Telefax: rotation speed and velocity during head collision. The 018 – 471 30 00 applicability of this tool is a predefined set of boxing match videos. The methodology however, can be Hemsida: extended for the analysis of different kinds of moving http://www.teknat.uu.se/student head objects. The user of the analysis tool should have basic ideas of how the different functionalities of the tool work and how to handle it properly. This project is a computer programming work which involves the study of the background, the study of methodology and a programming phase which gives result of the study. -

Textures
CS7029 Animation Project Part 2: Materials and Textures Anton Gerdelan <[email protected]> materials with shaders Materials What is a “material” exactly? ● container/abstraction ○ shaders: define drawing style ○ textures: (images) https://www.blender.org/manual/render/blender_render/materials/introduction.html Shaders: style of rendering ● mini programmes ● written in a shader programming language ○ e.g. OpenGL has GLSL shaders ○ Blender has its own too ● define lighting interactions: ○ reflection, refraction ○ surface colours ● sample colours from textures a material using the “glass” shader. “BSDF” = affected by lighting ● inputs and [colour] outputs tweak some settings “IOR” = index of refraction shader output is linked to surface colour this is a wonderful tutorial for materials in cycles https://www.youtube.com/watch?v=YR-m5J6vDt8 Blender Tutorial - Rendering with Cycles by Sardi Pax and for reducing noisy ‘fireflies’ further: http://www.blenderguru.com/articles/7-ways-get-rid-fireflies/ materials summary ● materials combine base properties, shaders, [and optional textures] ○ older Blender had properties based on Phong’s lighting model ■ diffuse (Lambert) colour ■ specular colour ○ newer Blender has properties based on “physically based rendering” ■ base colour ■ roughness ● style of rendering / colouring / lighting ● limited control in object properties pane ● more noodly visual control in the node editor mode ○ can mix outputs from multiple shaders to final surface colour ● i don’t use blender materials for games models textures Background to Textures and Texture Mapping Academic and technical history (insight you won’t get this from tutorials) Texture mapping is about using images transformed in 3d Did anyone see Ed Catmull talk here before the Web Summit? (Walt Disney and Pixar Animation Studios) Why “textures?” - that is a weird name. -

Virtualdub Documentation.Pdf
VirtualDub documentation: Capture Video capture in Windows is hardly a walk in the park, so here's a few tips to keep you going. As usual, corrections are welcome. How fast of a CPU do I need? This is a hard question to answer, but I can tell you what I've been able to do: • Celeron 300 @ 450, Hauppauge WinTV, 44KHz 16-bit stereo PCM, 640x480 YUY2 at 29.97 fps compressed with PicVideo at quality 18, on-screen display disabled. • Pentium III 733, Hauppauge WinTV, 44KHz 16-bit stereo PCM, 640x480 YUY2 at 29.97 fps compressed with Huffyuv in Predict Median, Preview on. Note that these are with a 16-bit desktop. You will take a sizeable hit with a 24-bit or 32-bit desktop if you have Preview on. VirtualDub says I don't have a capture device, but I know I have one. VirtualDub needs a Video for Windows capture driver to capture. Most Firewire (DV) devices do not provide a VFW driver, and thus cannot be used by VirtualDub at all. Also, ATI appears to be shipping their current devices with a WDM (Windows Driver Model) driver only; this can be used indirectly by VirtualDub through a Microsoft wrapper, but it is crippled in functionality and it also appears that the wrapper is buggy. The wrapper will show up as "Microsoft WDM Image Capture (Win32)." If it works for you, great. I can capture DV through VirtualDub, but I get the audio out of my sound card instead. Video for Windows only captures audio through a sound device; with DV, the audio is interleaved with the video itself. -

Time-Lapse Movie Guide
Conversion of time-lapse sequences of still images to movie files (step by step guide, freeware solutions) Martin Setvák [email protected] Version: 31 March 2008 Preface I was motivated to write this “guide” by two factors. First is the recent (March 2008) “Ricoh-boom” among my friends and colleagues, namely meteorologists and weather enthusiasts, so this should help them process their time-lapse sequences made by these cameras (but not only these). The second reason is that occasionally I see a time-lapse movie of rather lower technical quality (which applies also to some of my older movies), which could have been much better if the author knew how to do it and what settings to use when processing the sequence he or she has made. Some of the steps described below might appear so obvious that most of you will ask “why does he describes this - it is so clear that he might have skipped this” … but I was trying to help even the beginners, so excuse the “step by step” nature of the guide. At the beginning I wanted to prepare a simple web page, but while preparing the screenshots and realizing that this was going to be much larger than I originally thought, I changed my mind and decided to prepare a PDF version instead. The advantage of this form is that you can download or print it, and have it at your hand whenever needed. This guide describes the software I prefer and use; all of it is freeware (for non-commercial use). Obviously, there are other alternatives - perhaps even some which can handle all three steps described below at once - without a need to jump from one software package to another to complete all the tasks.