And Associated Sulfide and Sulfosalt Minerals
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Stibnite Gold Project
STIBNITE GOLD PROJECT PAYETTE & BOISE NATIONAL FORESTS INTERMOUNTAIN REGION August 22, 2018 STIBNITE MINING DISTRICT, PAYETTE NATIONAL FOREST, KRASSEL RANGER DISTRICT Regarding Locatable Minerals , 36 CFR 228, Subpart A: requires the Forest Service to: "Where conflicting interests Respond to a mining plan, evaluate that plan must be reconciled, the Consider requirements to minimize adverse effects to the extent feasible question shall always be Comply with applicable laws, regulations and answered from the stand- standards for environmental protection point of the greatest good of Assure appropriate reclamation Further respond by following National Environmental the greatest number in the Policy Act (NEPA) processes. long run." - James Wilson, Secretary Per 36 CFR 228.4, the Forest Service is required to take of Agriculture, 1905 in a letter to Gifford action to ensure that “operations are conducted so as, Pinchot, 1st Chief of the Forest Service. where feasible, to minimize adverse environmental impacts on National Forest surface resources. 1 Table of Contents Project Context STIBNITE GOLD Project Location 4 Mining History at Stibnite 5 PROJECT Who is Midas Gold 6 Stibnite Plan of Operations PAYETTE & BOISE NATIONAL Proposed Mining Plan of FOREST - INTERMOUNTAIN REGION Operations 7 Open Pit Mining 8 Anadromous Fish - Tunnel 9 Inventoried Roadless Areas - Why is this project of interest to many Access and Powerline 10 people and organizations? Facilities During Mining Operations 10 5 million recoverable ounces of gold x -
The Crystal Structure of Gillulyite, TI2(As,Sb)Ssm from the Mercur Gold Deposit, Tooele County, Utah, U.S.A
American Mineralogist, Volume 80, pages 394-399, 1995 The crystal structure of gillulyite, TI2(As,Sb)sSm from the Mercur gold deposit, Tooele County, Utah, U.S.A. FRANKLIN F. FOIT, JR. Department of Geology, Washington State University, Pullman, Washington 99164, U.S.A. PAUL D. ROBINSON Department of Geology, Southern Illinois University, Carbondale, Illinois 62901, U.S.A. JAMES R. WILSON Department of Geology, Weber State University, Ogden, Utah 84408-2507, U.S.A. ABSTRACT Gillulyite, TI2(AsH ,SbH )8S13,is monoclinic with space group P2/n and a = 9.584(3), b = 5.679(2), c = 21.501(6) A, ~ = 100.07(2)°, V = 1152.2(7) A3, and Z = 2, The average structure was determined using direct methods and refined to a final residual of 0.046 using 930 reflections. The structure consists of TI-As-S- and As-S-bearing sheets, each present statistically 50% of the time. Four sheets, linked together by Tl-S, As-S, and S-S bonds, are stacked parallel to (00 I), yielding the 21-A c-axis repeat of the average unit cell. The Tll atom within the TI-As-S sheet is coordinated by six S nearest neighbors, which form a distorted trigonal prism. The partially occupied Tl2 position between the sheets is split symmetrically about the twofold axis with a separation of 1.28 A and equal but partial occupancies of25%. The disordering of the Tl2 position and extreme distortion of its coordination polyhedron is probably the result of the Tl+ 6s2lone pair effect. The mean bond distances for the AsS3pyramids range from 2.263 to 2.319 A. -

Mineral Processing
Mineral Processing Foundations of theory and practice of minerallurgy 1st English edition JAN DRZYMALA, C. Eng., Ph.D., D.Sc. Member of the Polish Mineral Processing Society Wroclaw University of Technology 2007 Translation: J. Drzymala, A. Swatek Reviewer: A. Luszczkiewicz Published as supplied by the author ©Copyright by Jan Drzymala, Wroclaw 2007 Computer typesetting: Danuta Szyszka Cover design: Danuta Szyszka Cover photo: Sebastian Bożek Oficyna Wydawnicza Politechniki Wrocławskiej Wybrzeze Wyspianskiego 27 50-370 Wroclaw Any part of this publication can be used in any form by any means provided that the usage is acknowledged by the citation: Drzymala, J., Mineral Processing, Foundations of theory and practice of minerallurgy, Oficyna Wydawnicza PWr., 2007, www.ig.pwr.wroc.pl/minproc ISBN 978-83-7493-362-9 Contents Introduction ....................................................................................................................9 Part I Introduction to mineral processing .....................................................................13 1. From the Big Bang to mineral processing................................................................14 1.1. The formation of matter ...................................................................................14 1.2. Elementary particles.........................................................................................16 1.3. Molecules .........................................................................................................18 1.4. Solids................................................................................................................19 -

40 Common Minerals and Their Uses
40 Common Minerals and Their Uses Aluminum Beryllium The most abundant metal element in Earth’s Used in the nuclear industry and to crust. Aluminum originates as an oxide called make light, very strong alloys used in the alumina. Bauxite ore is the main source aircraft industry. Beryllium salts are used of aluminum and must be imported from in fluorescent lamps, in X-ray tubes and as Jamaica, Guinea, Brazil, Guyana, etc. Used a deoxidizer in bronze metallurgy. Beryl is in transportation (automobiles), packaging, the gem stones emerald and aquamarine. It building/construction, electrical, machinery is used in computers, telecommunication and other uses. The U.S. was 100 percent products, aerospace and defense import reliant for its aluminum in 2012. applications, appliances and automotive and consumer electronics. Also used in medical Antimony equipment. The U.S. was 10 percent import A native element; antimony metal is reliant in 2012. extracted from stibnite ore and other minerals. Used as a hardening alloy for Chromite lead, especially storage batteries and cable The U.S. consumes about 6 percent of world sheaths; also used in bearing metal, type chromite ore production in various forms metal, solder, collapsible tubes and foil, sheet of imported materials, such as chromite ore, and pipes and semiconductor technology. chromite chemicals, chromium ferroalloys, Antimony is used as a flame retardant, in chromium metal and stainless steel. Used fireworks, and in antimony salts are used in as an alloy and in stainless and heat resisting the rubber, chemical and textile industries, steel products. Used in chemical and as well as medicine and glassmaking. -

Using 2D Gis to Assist 3D Modelling of the Zarshuran Gold Deposit, Iran
Asadi, Hooshang USING 2D GIS TO ASSIST 3D MODELLING OF THE ZARSHURAN GOLD DEPOSIT, IRAN * Hooshang ASADI HARONI, Edmund SIDES, Kiiza NGONZI International Institute for Aerospace Survey and Earth Sciences (ITC), The Netherlands * Ministry of Higher Education, Tehran, Iran harouni @itc.nl [email protected] Technical Commission Session Themes TC VII-8 KEY WORDS: Spatial data, Integration, Geology, Geophysics, GIS, Data mining, Information extraction ABSTRACT The Zarshuran gold deposit in NW Iran is an area of historic mining for gold and arsenic with considerable potential for discovery of economic gold mineralisation. Geological, geochemical and geophysical data, collected by the Ministry of Mines and Metals were compiled and analysed in a 2-dimensional (2D) GIS. This resulted in the definition of major structural features, and lithological units, that control the gold mineralisation. Spatial modelling and interpretation of the geochemical and geophysical data showed that the mineralization is mainly controlled by chemically-reactive Precambrian carbonates and black shales, extending in a NW-SE direction, and also by NE-SW high angle faults and their intersections with NW-SE structures. The results obtained, from the 2D GIS analysis, were used in the initial phase of the construction and validation of the 3-dimensional (3D) models used for resource estimation. Comparison of the statistical analyses of geochemical data in soils and in drillcore indicated enhanced concentrations of gold in soils at surface, due to residual enrichment. An enrichment relationship was established based on interpretation of the cumulative frequency plots for gold in soil and drillcore samples. Based on this relationship the gold anomalies interpreted from the soil geochemical data were used to infer a resource potential, to a depth of 200m below surface, of 10Mt at an average grade of 0.2 g/t gold. -

Environmental Exposure of Thallium and Potential Health Risk in an Area of High Natural Concentrations of Thallium: Southwest Guizhou, China
Environmental Exposure and Health 367 Environmental exposure of thallium and potential health risk in an area of high natural concentrations of thallium: southwest Guizhou, China T. Xiao1, L. He1,3, J. Guha2, J. Lin1 & J. Chen1 1State Key Laboratory of Environmental Geochemistry, Institute of Geochemistry, Chinese Academy of Sciences, People’s Republic of China 2Sciences de la Terre, Université du Québec à Chicoutimi, Canada 3Graduate School of Chinese Academy of Sciences, Beijing, People’s Republic of China Abstract Little is known in the literature about thallium (Tl) exposure from naturally occurring Tl contamination. This paper draws attention to the potential health risk posed by high concentrations of naturally occurring Tl in the environment. The inhabitants of a rural area of southwest Guizhou Province, China, reside within a natural Tl accumulated environment resulting from the Tl-rich sulfide mineralization, and they face a severe Tl exposure in their daily lives. The daily intake 1.9 mg Tl from the consumed food crops was estimated for a local adult inhabitant of Lanmuchang. High Tl concentrations were detected in urines of the local residents. Measured urinary Tl levels are as high as 2.51-2,668 µg/L, surpassing the accepted world urine Tl level <1 mg/L for “non-exposed” humans. However, there is a positive relationship between the extent of Tl exposure from Tl in soil and crops in the immediate environment and the levels of Tl detected in urine. This study has been able to identify that the elevated urinary Tl levels are mainly attributable to Tl accumulation in locally grown vegetables acquiring Tl from natural sources in the local soils. -

The Importance of Minerals in Coal As the Hosts of Chemical Elements: a Review
The importance of minerals in coal as the hosts of chemical elements: A review Robert B. Finkelmana,b, Shifeng Daia,c,*, David Frenchd a State Key Laboratory of Coal Resources and Safe Mining, China University of Mining and Technology, China b University of Texas at Dallas, Richardson, TX 75080, USA c College of Geoscience and Survey Engineering, China University of Mining and Technology (Beijing), Beijing 100083, China d PANGEA Research Centre, School of Biological, Earth and Environmental Sciences, University of New South Wales, Sydney, NSW 2052, Australia *, Corresponding author: [email protected]; [email protected] Abstract Coal is a complex geologic material composed mainly of organic matter and mineral matter, the latter including minerals, poorly crystalline mineraloids, and elements associated with non- mineral inorganics. Among mineral matter, minerals play the most significant role in affecting the utilization of coal, although, in low rank coals, the non-mineral elements may also be significant. Minerals in coal are often regarded as a nuisance being responsible for most of the problems arising during coal utilization, but the minerals are also seen as a potentially valuable source of critical metals and may also, in some cases, have a beneficial effect in coal gasification and liquefaction. With a few exceptions, minerals are the major hosts of the vast majority of elements present in coal. In this review paper, we list more than 200 minerals that have been identified in coal and its low temperature ash, although the validity of some of these minerals has not been confirmed. Base on chemical compositions, minerals found in coal can be classified into silicate, sulfide and selenide, phosphate, carbonate, sulfate, oxide and hydroxide, and others. -

~Ui&£R5itt! of J\Rij!Oua
Minerals and metals of increasing interest, rare and radioactive minerals Authors Moore, R.T. Rights Arizona Geological Survey. All rights reserved. Download date 06/10/2021 17:57:35 Link to Item http://hdl.handle.net/10150/629904 Vol. XXIV, No.4 October, 1953 ~ui&£r5itt! of J\rij!oua ~ul1etiu ARIZONA BUREAU OF MINES MINERALS AND METALS OF INCREASING INTEREST RARE AND RADIOACTIVE MINERALS By RICHARD T. MOORE ARIZONA BUREAU OF MINES MINERAL TECHNOLOGY SERIES No. 47 BULLETIN No. 163 THIRTY CENTS (Free to Residents of Arizona) PUBLISHED BY ~tti£ll~r5itt! of ~rh!Omt TUCSON, ARIZONA TABLE OF CONTENTS INTRODUCTION 5 Acknowledgments 5 General Features 5 BERYLLIUM 7 General Features 7 Beryllium Minerals 7 Beryl 7 Phenacite 8 Gadolinite 8 Helvite 8 Occurrence 8 Prices and Possible Buyers ,........................................ 8 LITHIUM 9 General Features 9 Lithium Minerals 9 Amblygonite 9 Spodumene 10 Lepidolite 10 Triphylite 10 Zinnwaldite 10 Occurrence 10 Prices and Possible Buyers 10 CESIUM AND RUBIDIUM 11 General Features 11 Cesium and Rubidium Minerals 11 Pollucite ..................•.........................................................................., 11 Occurrence 12 Prices and Producers 12 TITANIUM 12 General Features 12 Titanium Minerals 13 Rutile 13 Ilmenite 13 Sphene 13 Occurrence 13 Prices and Buyers 14 GALLIUM, GERMANIUM, INDIUM, AND THALLIUM 14 General Features 14 Gallium, Germanium, Indium and Thallium Minerals 15 Germanite 15 Lorandite 15 Hutchinsonite : 15 Vrbaite 15 Occurrence 15 Prices and Producers ~ 16 RHENIUM 16 -

Thallium Mobility in Mining Wastes at the Crven Dol Locality, Allchar Deposit, North Macedonia
EGU2020-4959, updated on 25 Sep 2021 https://doi.org/10.5194/egusphere-egu2020-4959 EGU General Assembly 2020 © Author(s) 2021. This work is distributed under the Creative Commons Attribution 4.0 License. Thallium mobility in mining wastes at the Crven Dol locality, Allchar deposit, North Macedonia Tamara Đorđević1, Uwe Kolitsch1,2, Petr Drahota3, Magdaléna Knappová3, Juraj Majzlan4, Stefan Kiefer4, Tomáš Mikuš5, Goran Tasev6, Todor Serafimovski6, Ivan Boev6, and Blažo Boev6 1Institut für Mineralogie und Kristallographie, Universität Wien, Althanstr. 14, A-1090 Wien, Austria 2Mineralogisch-Petrographische Abteilung, Naturhistorisches Museum, Burgring 7, A-1010 Wien, Austria 3Institute of Geochemistry, Mineralogy and Mineral Resources, Faculty of Science, Charles University, Albertov 6, 128 43 Prague 2, Czech Republic 4Institute of Geosciences, Department of Mineralogy, Friedrich-Schiller-Universität, Carl-Zeiss-Promenade 10, 07745 Jena, Germany 5Earth Science Institute, Slovak Academy of Sciences, Geological Division, Ďumbierska 1, 974 01 Banská Bystrica, Slovakia 6Department of Mineral Deposits, Faculty of Natural Sciences, University “Goce Delčev”-Štip, Goce Delčev 89, 2000 Štip, North Macedonia In order to better understand the environmental behaviour of thallium, we have chosen the abandoned As–Sb–Tl–Au Allchar deposit (North Macedonia) with unique mineral composition and high thallium grades of the ore. We used pore water analyses, selective extractions, single-crystal and powder X-ray diffraction (PXRD), SEM-EDS, electron microprobe analysis (EMPA), and Raman spectroscopy to determine the distribution and speciation of thallium in waste dump material at the Tl-rich Crven Dol locality in the northern part of the Allchar deposit. PXRD studies showed that the various solid waste samples are comprised mostly of carbonates (dolomite and calcite), gypsum, quartz, muscovite, kaolinite-group minerals followed by orpiment, realgar, pyrite, marcasite, lorandite, and various iron and calcium arsenates and iron (hydro)oxides, both amorphous and crystalline. -

XIII 0609.Pdf
XIII_0609 N. Jb. Miner. Abh. Stuttgart, Oktober 1994 Possibilities of concentrating the thallium minerallorandite from the Allchar deposit, Crven Dol region By Blagoja Petrov, Donka Andonova, Trajce Stafilov and Tome Novakovski, Skopje With 3 figures and 3 tables in the text PETROV, B., ANDONOVA, D. STAHLOV, T. & NOVAKOVSKJ, T.: Possibilities of concentrating the thallium mineral from the Allchar deposit , Crven Dol region . N. jb. Miner. Abh. 167: 413-420; Stuttgart 1994. Abstract: The paper presents results obta ined by laboratory testings on th e possibilities of concentrating the thallium minerallorandite from ore in the Allchar deposit, Crven Dol region. For concentrating the lorandite from ore, stand ard methods were used: gravity concentrating in heavy liquids, gravity concentrating on a concentrating table, high intensity magnetic separation by an isodynamic separator "Fr ant z" as well as their combinations. From the -preconcentrates obtained, pure grains of lorandire were sepa rated by picking of individual grains. Ke y w 0 r d s: Thallium minerals; lorandite minera l; concentrating lor andite; gravity concentrating; high intensity magnetic separation. Introduction Thallium belongs to the rare metals. According to Arence, the average thallium content in the Earth's crust amounts to about 0.003 %. It mostly appears in a form of solid solution in the lattices of sulfide minerals. Thal lium, very seldom, forms its own minerals. Most frequently, it is in para genesis with the sulfide minerals particularly galenite, sphalerite, mar casite and pyrite. Usually, it can be obtained as a by-product when roasting pyrite for the production of sulphuric acid and smelting of lead and zinc. -
Antimony Data Sheet
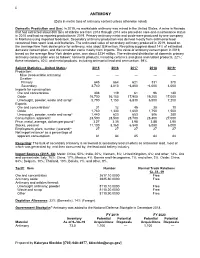
22 ANTIMONY (Data in metric tons of antimony content unless otherwise noted) Domestic Production and Use: In 2019, no marketable antimony was mined in the United States. A mine in Nevada that had extracted about 800 tons of stibnite ore from 2013 through 2014 was placed on care-and-maintenance status in 2015 and had no reported production in 2019. Primary antimony metal and oxide were produced by one company in Montana using imported feedstock. Secondary antimony production was derived mostly from antimonial lead recovered from spent lead-acid batteries. The estimated value of secondary antimony produced in 2019, based on the average New York dealer price for antimony, was about $34 million. Recycling supplied about 14% of estimated domestic consumption, and the remainder came mostly from imports. The value of antimony consumption in 2019, based on the average New York dealer price, was about $234 million. The estimated distribution of domestic primary antimony consumption was as follows: nonmetal products, including ceramics and glass and rubber products, 22%; flame retardants, 40%; and metal products, including antimonial lead and ammunition, 39%. Salient Statistics—United States: 2015 2016 2017 2018 2019e Production: Mine (recoverable antimony) — — — — — Smelter: Primary 645 664 621 331 370 Secondary 3,740 3,810 e3,800 e4,000 4,000 Imports for consumption: Ore and concentrates 308 119 61 96 140 Oxide 16,700 16,100 17,900 19,200 17,000 Unwrought, powder, waste and scrap1 5,790 7,150 6,830 6,500 7,200 Exports: Ore and concentrates1 31 -

Low Temperature Aqueous Solubility of Fluorite at Temperatures of 5, 25, and 50 Oc and Ionic Strengths up to 0.72M
LOW TEMPERATURE AQUEOUS SOLUBILITY OF FLUORITE AT TEMPERATURES OF 5, 25, AND 50 OC AND IONIC STRENGTHS UP TO 0.72M by Richard L. Henry A thesis submitted to the Faculty and Board of Trustees of the Colorado School of Mines in partial fulfillment of the requirement for the degree of Doctor of Philosophy (Geochemistry) Golden, Colorado Date __________________________ Signed: ______________________________ Richard L. Henry Signed: ______________________________ Dr. Wendy J. Harrison Thesis Advisor Golden, Colorado Date __________________________ Signed: ______________________________ Dr. M. Stephen Enders Professor and Head Department of Geology and Geological Engineering ii ABSTRACT Historical fluorite solubility research found that fluorite solubilities varied over three orders-of-magnitude and appear to be inconsistent with several recently determined fluorite solubilities. This research involved a comprehensive series of solubility experiments run over a period of three years using nine natural fluorites and one synthetic fluorite to determine their solubility in low temperature (5, 25, and 50oC) aqueous solutions and ionic strengths ranging from near zero to 0.72M. Equilibrium was approached from both undersaturated and supersaturated initial conditions. The fluorites were chemically and physically characterized using laboratory analyses, thin section petrography, UV fluorescence, and x-ray diffraction. The fluorite samples were generally found to have similar chemical and physical properties. The chemical composition was approximately stoichiometric, with a mean calcium concentration of 51.6 ± 0.6 wt % and mean fluorine concentration of 50.2 ± 0.7 wt %. Fluorite unit cell edge lengths, volumes, and densities averaged 5.4630 ± 0.0007 Å, 163.04 ± 0.06 Å3, and 3.181 ± 0.009 g/cm3, respectively.