Ancistrocladaceae
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

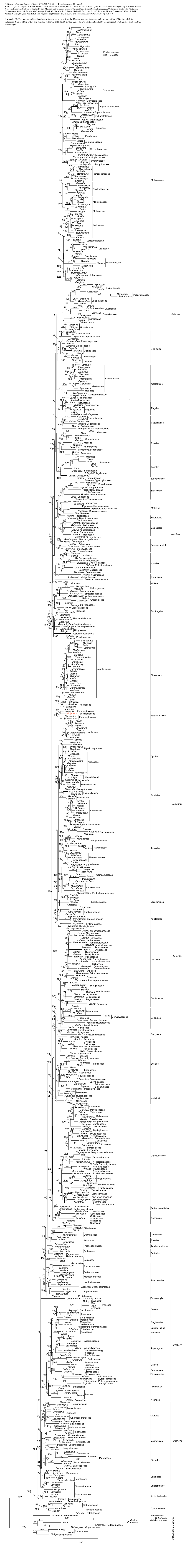
Toward a Resolution of Campanulid Phylogeny, with Special Reference to the Placement of Dipsacales
TAXON 57 (1) • February 2008: 53–65 Winkworth & al. • Campanulid phylogeny MOLECULAR PHYLOGENETICS Toward a resolution of Campanulid phylogeny, with special reference to the placement of Dipsacales Richard C. Winkworth1,2, Johannes Lundberg3 & Michael J. Donoghue4 1 Departamento de Botânica, Instituto de Biociências, Universidade de São Paulo, Caixa Postal 11461–CEP 05422-970, São Paulo, SP, Brazil. [email protected] (author for correspondence) 2 Current address: School of Biology, Chemistry, and Environmental Sciences, University of the South Pacific, Private Bag, Laucala Campus, Suva, Fiji 3 Department of Phanerogamic Botany, The Swedish Museum of Natural History, Box 50007, 104 05 Stockholm, Sweden 4 Department of Ecology & Evolutionary Biology and Peabody Museum of Natural History, Yale University, P.O. Box 208106, New Haven, Connecticut 06520-8106, U.S.A. Broad-scale phylogenetic analyses of the angiosperms and of the Asteridae have failed to confidently resolve relationships among the major lineages of the campanulid Asteridae (i.e., the euasterid II of APG II, 2003). To address this problem we assembled presently available sequences for a core set of 50 taxa, representing the diver- sity of the four largest lineages (Apiales, Aquifoliales, Asterales, Dipsacales) as well as the smaller “unplaced” groups (e.g., Bruniaceae, Paracryphiaceae, Columelliaceae). We constructed four data matrices for phylogenetic analysis: a chloroplast coding matrix (atpB, matK, ndhF, rbcL), a chloroplast non-coding matrix (rps16 intron, trnT-F region, trnV-atpE IGS), a combined chloroplast dataset (all seven chloroplast regions), and a combined genome matrix (seven chloroplast regions plus 18S and 26S rDNA). Bayesian analyses of these datasets using mixed substitution models produced often well-resolved and supported trees. -

Griselinia Littoralis Broadway Mint
Griselinia littoralis Broadway Mint Griselinia littoralis Broadway Mint Botanical Name: Griselinia littoralis Broadway Mint Common Names: Kapuka, New Zealand Broadleaf, Native: No Foliage Type: Evergreen Plant Type: Hedging / Screening, Shrubs Plant Habit: Dense, Shrub Like, Upright, Upright Narrow Description: Lush, emerald green glossy wavy-leaved tall dense growing shrub perfect for a compact, low hedge. Naturally growing to 4m, the Griselinia hedges well 1-4m tall. Tough, wind tolerant and quick growing, this is also a great choice for screens and coastal plantings. Mature Height: 2-4m Position: Full Sun, Semi Shade Mature Width: 1-2m Soil Type: Loam, Sandy, Well Drained Family Name: TBA Landscape Use(s): Borders / Shrubbery, Coastal Garden, Courtyard, Foliage Feature / Colour, Formal Garden, Hedging / Screening, Park And Gardens, Wind Origin: PacificIslands Break, Container / Pot Characteristics: Pest & Diseases: Foliage Colours: Green Generally trouble free Flower Colours: Insignificant Flower Fragrant: No Cultural Notes: Flowering Season: N/A Fruit: Insignificant Plant Care: Requirements: Annual slow release fertiliser, Keep moist during dry periods, Liquid feed Growth Rate: Moderate Maintenance Level: Low Water Usage: Medium / Moderate Tolerances: Drought: Medium / Moderate Frost: Moderate Wind: Moderate Disclaimer: Information and images provided is to be used as a guide only. While every reasonable effort is made to ensure accuracy and relevancy of all information, any decisions based on this information are the sole responsibility of the viewer. Call 1300 787 401 plantmark.com.au. -

Guide to the Flora of the Carolinas, Virginia, and Georgia, Working Draft of 17 March 2004 -- LILIACEAE
Guide to the Flora of the Carolinas, Virginia, and Georgia, Working Draft of 17 March 2004 -- LILIACEAE LILIACEAE de Jussieu 1789 (Lily Family) (also see AGAVACEAE, ALLIACEAE, ALSTROEMERIACEAE, AMARYLLIDACEAE, ASPARAGACEAE, COLCHICACEAE, HEMEROCALLIDACEAE, HOSTACEAE, HYACINTHACEAE, HYPOXIDACEAE, MELANTHIACEAE, NARTHECIACEAE, RUSCACEAE, SMILACACEAE, THEMIDACEAE, TOFIELDIACEAE) As here interpreted narrowly, the Liliaceae constitutes about 11 genera and 550 species, of the Northern Hemisphere. There has been much recent investigation and re-interpretation of evidence regarding the upper-level taxonomy of the Liliales, with strong suggestions that the broad Liliaceae recognized by Cronquist (1981) is artificial and polyphyletic. Cronquist (1993) himself concurs, at least to a degree: "we still await a comprehensive reorganization of the lilies into several families more comparable to other recognized families of angiosperms." Dahlgren & Clifford (1982) and Dahlgren, Clifford, & Yeo (1985) synthesized an early phase in the modern revolution of monocot taxonomy. Since then, additional research, especially molecular (Duvall et al. 1993, Chase et al. 1993, Bogler & Simpson 1995, and many others), has strongly validated the general lines (and many details) of Dahlgren's arrangement. The most recent synthesis (Kubitzki 1998a) is followed as the basis for familial and generic taxonomy of the lilies and their relatives (see summary below). References: Angiosperm Phylogeny Group (1998, 2003); Tamura in Kubitzki (1998a). Our “liliaceous” genera (members of orders placed in the Lilianae) are therefore divided as shown below, largely following Kubitzki (1998a) and some more recent molecular analyses. ALISMATALES TOFIELDIACEAE: Pleea, Tofieldia. LILIALES ALSTROEMERIACEAE: Alstroemeria COLCHICACEAE: Colchicum, Uvularia. LILIACEAE: Clintonia, Erythronium, Lilium, Medeola, Prosartes, Streptopus, Tricyrtis, Tulipa. MELANTHIACEAE: Amianthium, Anticlea, Chamaelirium, Helonias, Melanthium, Schoenocaulon, Stenanthium, Veratrum, Toxicoscordion, Trillium, Xerophyllum, Zigadenus. -

Floral Symmetry Affects Speciation Rates in Angiosperms Risa D
Received 25 July 2003 Accepted 13 November 2003 Published online 16 February 2004 Floral symmetry affects speciation rates in angiosperms Risa D. Sargent Department of Zoology, University of British Columbia, 6270 University Boulevard, Vancouver, British Columbia V6T 1Z4, Canada ([email protected]) Despite much recent activity in the field of pollination biology, the extent to which animal pollinators drive the formation of new angiosperm species remains unresolved. One problem has been identifying floral adaptations that promote reproductive isolation. The evolution of a bilaterally symmetrical corolla restricts the direction of approach and movement of pollinators on and between flowers. Restricting pollin- ators to approaching a flower from a single direction facilitates specific placement of pollen on the pollin- ator. When coupled with pollinator constancy, precise pollen placement can increase the probability that pollen grains reach a compatible stigma. This has the potential to generate reproductive isolation between species, because mutations that cause changes in the placement of pollen on the pollinator may decrease gene flow between incipient species. I predict that animal-pollinated lineages that possess bilaterally sym- metrical flowers should have higher speciation rates than lineages possessing radially symmetrical flowers. Using sister-group comparisons I demonstrate that bilaterally symmetric lineages tend to be more species rich than their radially symmetrical sister lineages. This study supports an important role for pollinator- mediated speciation and demonstrates that floral morphology plays a key role in angiosperm speciation. Keywords: reproductive isolation; pollination; sister group comparison; zygomorphy 1. INTRODUCTION The importance of pollinator-mediated selection in angiosperms is well supported by theory (Kiester et al. -

Flowering Plant Families of Northwestern California: a Tabular Comparison
Humboldt State University Digital Commons @ Humboldt State University Botanical Studies Open Educational Resources and Data 12-2019 Flowering Plant Families of Northwestern California: A Tabular Comparison James P. Smith Jr Humboldt State University, [email protected] Follow this and additional works at: https://digitalcommons.humboldt.edu/botany_jps Part of the Botany Commons Recommended Citation Smith, James P. Jr, "Flowering Plant Families of Northwestern California: A Tabular Comparison" (2019). Botanical Studies. 95. https://digitalcommons.humboldt.edu/botany_jps/95 This Flora of Northwest California-Regional is brought to you for free and open access by the Open Educational Resources and Data at Digital Commons @ Humboldt State University. It has been accepted for inclusion in Botanical Studies by an authorized administrator of Digital Commons @ Humboldt State University. For more information, please contact [email protected]. FLOWERING PLANT FAMILIES OF NORTHWESTERN CALIFORNIA: A TABULAR COMPARISON James P. Smith, Jr. Professor Emeritus of Botany Department of Biological Sciences Humboldt State University December 2019 Scientific Name Habit Leaves Sexuality • Floral Formula Common Name Fruit Type • Comments Aceraceae TSV SC:O U-m [P] • K 4-5 C 4-5 A 4-10 G (2) Maple Paired samaras • leaves often palmately lobed Acoraceae H S:A U-m • P 3+3 A 6 or G (3) Sweet Flag Berry • aquatic; aromatic rhizomes Aizoaceae HS S:AO B • P [3] 5 [8] A 0-4 Gsi (2-5-4) Ice Plant Capsule (berry-like) • fleshy; stamens divided, petaloid Alismataceae -

Outline of Angiosperm Phylogeny
Outline of angiosperm phylogeny: orders, families, and representative genera with emphasis on Oregon native plants Priscilla Spears December 2013 The following listing gives an introduction to the phylogenetic classification of the flowering plants that has emerged in recent decades, and which is based on nucleic acid sequences as well as morphological and developmental data. This listing emphasizes temperate families of the Northern Hemisphere and is meant as an overview with examples of Oregon native plants. It includes many exotic genera that are grown in Oregon as ornamentals plus other plants of interest worldwide. The genera that are Oregon natives are printed in a blue font. Genera that are exotics are shown in black, however genera in blue may also contain non-native species. Names separated by a slash are alternatives or else the nomenclature is in flux. When several genera have the same common name, the names are separated by commas. The order of the family names is from the linear listing of families in the APG III report. For further information, see the references on the last page. Basal Angiosperms (ANITA grade) Amborellales Amborellaceae, sole family, the earliest branch of flowering plants, a shrub native to New Caledonia – Amborella Nymphaeales Hydatellaceae – aquatics from Australasia, previously classified as a grass Cabombaceae (water shield – Brasenia, fanwort – Cabomba) Nymphaeaceae (water lilies – Nymphaea; pond lilies – Nuphar) Austrobaileyales Schisandraceae (wild sarsaparilla, star vine – Schisandra; Japanese -

Extreme Ecological Specialization in a Rainforest Mammal, the Bornean
bioRxiv preprint doi: https://doi.org/10.1101/2020.08.03.233999; this version posted August 3, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 1 2 3 4 Extreme ecological specialization in a rainforest mammal, 5 the Bornean tufted ground squirrel, Rheithrosciurus macrotis 6 7 8 Andrew J. Marshall1*, Erik Meijaard2, and Mark Leighton3 9 10 1Department of Anthropology, Department of Ecology and Evolutionary Biology, Program in the 11 Environment, and School for Environment and Sustainability, 101 West Hall, 1085 S. University 12 Ave, Ann Arbor, Michigan, 48109 USA. 13 2Borneo Futures, Block C, Unit C8, Second Floor, Lot 51461, Kg Kota Batu, Mukim Kota Batu, 14 BA 2711, Brunei Darussalam. 15 3Harvard University, 11 Divinity Ave, Cambridge, MA, 02138, U.S.A. 16 17 * Corresponding author 18 E-mail: [email protected] (AJM) 1 bioRxiv preprint doi: https://doi.org/10.1101/2020.08.03.233999; this version posted August 3, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 19 Abstract 20 The endemic Bornean tufted ground squirrel, Rheithrosciurus macrotis, has attracted great 21 interest among biologists and the public recently. Nevertheless, we lack information on the most 22 basic aspects of its biology. -

Ultramafic Geocology of South and Southeast Asia
Galey et al. Bot Stud (2017) 58:18 DOI 10.1186/s40529-017-0167-9 REVIEW Open Access Ultramafc geoecology of South and Southeast Asia M. L. Galey1, A. van der Ent2,3, M. C. M. Iqbal4 and N. Rajakaruna5,6* Abstract Globally, ultramafc outcrops are renowned for hosting foras with high levels of endemism, including plants with specialised adaptations such as nickel or manganese hyperaccumulation. Soils derived from ultramafc regoliths are generally nutrient-defcient, have major cation imbalances, and have concomitant high concentrations of potentially phytotoxic trace elements, especially nickel. The South and Southeast Asian region has the largest surface occur- rences of ultramafc regoliths in the world, but the geoecology of these outcrops is still poorly studied despite severe conservation threats. Due to the paucity of systematic plant collections in many areas and the lack of georeferenced herbarium records and databased information, it is not possible to determine the distribution of species, levels of end- emism, and the species most threatened. However, site-specifc studies provide insights to the ultramafc geoecology of several locations in South and Southeast Asia. The geoecology of tropical ultramafc regions difers substantially from those in temperate regions in that the vegetation at lower elevations is generally tall forest with relatively low levels of endemism. On ultramafc mountaintops, where the combined forces of edaphic and climatic factors inter- sect, obligate ultramafc species and hyperendemics often occur. Forest clearing, agricultural development, mining, and climate change-related stressors have contributed to rapid and unprecedented loss of ultramafc-associated habitats in the region. The geoecology of the large ultramafc outcrops of Indonesia’s Sulawesi, Obi and Halmahera, and many other smaller outcrops in South and Southeast Asia, remains largely unexplored, and should be prioritised for study and conservation. -

Plant Life of Western Australia
INTRODUCTION The characteristic features of the vegetation of Australia I. General Physiography At present the animals and plants of Australia are isolated from the rest of the world, except by way of the Torres Straits to New Guinea and southeast Asia. Even here adverse climatic conditions restrict or make it impossible for migration. Over a long period this isolation has meant that even what was common to the floras of the southern Asiatic Archipelago and Australia has become restricted to small areas. This resulted in an ever increasing divergence. As a consequence, Australia is a true island continent, with its own peculiar flora and fauna. As in southern Africa, Australia is largely an extensive plateau, although at a lower elevation. As in Africa too, the plateau increases gradually in height towards the east, culminating in a high ridge from which the land then drops steeply to a narrow coastal plain crossed by short rivers. On the west coast the plateau is only 00-00 m in height but there is usually an abrupt descent to the narrow coastal region. The plateau drops towards the center, and the major rivers flow into this depression. Fed from the high eastern margin of the plateau, these rivers run through low rainfall areas to the sea. While the tropical northern region is characterized by a wet summer and dry win- ter, the actual amount of rain is determined by additional factors. On the mountainous east coast the rainfall is high, while it diminishes with surprising rapidity towards the interior. Thus in New South Wales, the yearly rainfall at the edge of the plateau and the adjacent coast often reaches over 100 cm. -

P020110307527551165137.Pdf
CONTENT 1.MESSAGE FROM DIRECTOR …………………………………………………………………………………………………………………………………………………… 03 2.ORGANIZATION STRUCTURE …………………………………………………………………………………………………………………………………………………… 05 3.HIGHLIGHTS OF ACHIEVEMENTS …………………………………………………………………………………………………………………………………………… 06 Coexistence of Conserve and Research----“The Germplasm Bank of Wild Species ” services biodiversity protection and socio-economic development ………………………………………………………………………………………………………………………………………………… 06 The Structure, Activity and New Drug Pre-Clinical Research of Monoterpene Indole Alkaloids ………………………………………… 09 Anti-Cancer Constituents in the Herb Medicine-Shengma (Cimicifuga L) ……………………………………………………………………………… 10 Floristic Study on the Seed Plants of Yaoshan Mountain in Northeast Yunnan …………………………………………………………………… 11 Higher Fungi Resources and Chemical Composition in Alpine and Sub-alpine Regions in Southwest China ……………………… 12 Research Progress on Natural Tobacco Mosaic Virus (TMV) Inhibitors…………………………………………………………………………………… 13 Predicting Global Change through Reconstruction Research of Paleoclimate………………………………………………………………………… 14 Chemical Composition of a traditional Chinese medicine-Swertia mileensis……………………………………………………………………………… 15 Mountain Ecosystem Research has Made New Progress ………………………………………………………………………………………………………… 16 Plant Cyclic Peptide has Made Important Progress ………………………………………………………………………………………………………………… 17 Progresses in Computational Chemistry Research ………………………………………………………………………………………………………………… 18 New Progress in the Total Synthesis of Natural Products ……………………………………………………………………………………………………… -

Rare Plants of Louisiana
Rare Plants of Louisiana Agalinis filicaulis - purple false-foxglove Figwort Family (Scrophulariaceae) Rarity Rank: S2/G3G4 Range: AL, FL, LA, MS Recognition: Photo by John Hays • Short annual, 10 to 50 cm tall, with stems finely wiry, spindly • Stems simple to few-branched • Leaves opposite, scale-like, about 1mm long, barely perceptible to the unaided eye • Flowers few in number, mostly born singly or in pairs from the highest node of a branchlet • Pedicels filiform, 5 to 10 mm long, subtending bracts minute • Calyx 2 mm long, lobes short-deltoid, with broad shallow sinuses between lobes • Corolla lavender-pink, without lines or spots within, 10 to 13 mm long, exterior glabrous • Capsule globe-like, nearly half exerted from calyx Flowering Time: September to November Light Requirement: Full sun to partial shade Wetland Indicator Status: FAC – similar likelihood of occurring in both wetlands and non-wetlands Habitat: Wet longleaf pine flatwoods savannahs and hillside seepage bogs. Threats: • Conversion of habitat to pine plantations (bedding, dense tree spacing, etc.) • Residential and commercial development • Fire exclusion, allowing invasion of habitat by woody species • Hydrologic alteration directly (e.g. ditching) and indirectly (fire suppression allowing higher tree density and more large-diameter trees) Beneficial Management Practices: • Thinning (during very dry periods), targeting off-site species such as loblolly and slash pines for removal • Prescribed burning, establishing a regime consisting of mostly growing season (May-June) burns Rare Plants of Louisiana LA River Basins: Pearl, Pontchartrain, Mermentau, Calcasieu, Sabine Side view of flower. Photo by John Hays References: Godfrey, R. K. and J. W. Wooten. -

Phylogeographical Structure of Liquidambar Formosana Hance Revealed by Chloroplast Phylogeography and Species Distribution Models
Article Phylogeographical Structure of Liquidambar formosana Hance Revealed by Chloroplast Phylogeography and Species Distribution Models 1,2, 1, 1 2 1 1, Rongxi Sun y , Furong Lin y, Ping Huang , Xuemin Ye , Jiuxin Lai and Yongqi Zheng * 1 State Key Laboratory of Tree Genetics and Breeding, Key Laboratory of Silviculture of the State Forestry Administration, Research Institute of Forestry, Chinese Academy of Forestry, Beijing 100091, China; [email protected] (R.S.); [email protected] (F.L.); [email protected] (P.H.); [email protected] (J.L.) 2 Jiangxi Provincial Key Laboratory of Silviculture, College of Forestry, Jiangxi Agricultural University, Nanchang 330045, China; [email protected] * Correspondence: [email protected]; Tel.: +86-10-6288-8565 These authors contributed equally to this work. y Received: 2 September 2019; Accepted: 29 September 2019; Published: 1 October 2019 Abstract: To understand the origin and evolutionary history, and the geographical and historical causes for the formation of the current distribution pattern of Lquidambar formosana Hance, we investigated the phylogeography by using chloroplasts DNA (cpDNA) non-coding sequences and species distribution models (SDM). Four cpDNA intergenic spacer regions were amplified and sequenced for 251 individuals from 25 populations covering most of its geographical range in China. A total of 20 haplotypes were recovered. The species had a high level of chloroplast genetic variation (Ht = 0.909 0.0192) and a significant phylogeographical structure (genetic differentiation takes into ± account distances among haplotypes (Nst) = 0.730 > population differentiation that does not consider distances among haplotypes (Gst) = 0.645; p < 0.05), whereas the genetic variation within populations (Hs = 0.323 0.0553) was low.