Materials Data Book
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

10-1 CHAPTER 10 DEFORMATION 10.1 Stress-Strain Diagrams And
EN380 Naval Materials Science and Engineering Course Notes, U.S. Naval Academy CHAPTER 10 DEFORMATION 10.1 Stress-Strain Diagrams and Material Behavior 10.2 Material Characteristics 10.3 Elastic-Plastic Response of Metals 10.4 True stress and strain measures 10.5 Yielding of a Ductile Metal under a General Stress State - Mises Yield Condition. 10.6 Maximum shear stress condition 10.7 Creep Consider the bar in figure 1 subjected to a simple tension loading F. Figure 1: Bar in Tension Engineering Stress () is the quotient of load (F) and area (A). The units of stress are normally pounds per square inch (psi). = F A where: is the stress (psi) F is the force that is loading the object (lb) A is the cross sectional area of the object (in2) When stress is applied to a material, the material will deform. Elongation is defined as the difference between loaded and unloaded length ∆푙 = L - Lo where: ∆푙 is the elongation (ft) L is the loaded length of the cable (ft) Lo is the unloaded (original) length of the cable (ft) 10-1 EN380 Naval Materials Science and Engineering Course Notes, U.S. Naval Academy Strain is the concept used to compare the elongation of a material to its original, undeformed length. Strain () is the quotient of elongation (e) and original length (L0). Engineering Strain has no units but is often given the units of in/in or ft/ft. ∆푙 휀 = 퐿 where: is the strain in the cable (ft/ft) ∆푙 is the elongation (ft) Lo is the unloaded (original) length of the cable (ft) Example Find the strain in a 75 foot cable experiencing an elongation of one inch. -

The Effect of Yield Strength on Inelastic Buckling of Reinforcing
Mechanics and Mechanical Engineering Vol. 14, No. 2 (2010) 247{255 ⃝c Technical University of Lodz The Effect of Yield Strength on Inelastic Buckling of Reinforcing Bars Jacek Korentz Institute of Civil Engineering University of Zielona G´ora Licealna 9, 65{417 Zielona G´ora, Poland Received (13 June 2010) Revised (15 July 2010) Accepted (25 July 2010) The paper presents the results of numerical analyses of inelastic buckling of reinforcing bars of various geometrical parameters, made of steel of various values of yield strength. The results of the calculations demonstrate that the yield strength of the steel the bars are made of influences considerably the equilibrium path of the compressed bars within the range of postyielding deformations Comparative diagrams of structural behaviour (loading paths) of thin{walled sec- tions under investigation for different strain rates are presented. Some conclusions and remarks concerning the strain rate influence are derived. Keywords: Reinforcing bars, inelastic buckling, yield strength, tensil strength 1. Introduction The impact of some exceptional loads, e.g. seismic loads, on a structure may re- sult in the occurrence of post{critical states. Therefore the National Standards regulations for designing reinforced structures on seismically active areas e.g. EC8 [15] require the ductility of a structure to be examined on a cross{sectional level, and additionally, the structures should demonstrate a suitable level of global duc- tility. The results of the examinations of members of reinforced concrete structures show that inelastic buckling of longitudinal reinforcement bars occurs in the state of post{critical deformations, [1, 2, 4, 7], and in some cases it occurs yet within the range of elastic deformations [8]. -

Lecture 13: Earth Materials
Earth Materials Lecture 13 Earth Materials GNH7/GG09/GEOL4002 EARTHQUAKE SEISMOLOGY AND EARTHQUAKE HAZARD Hooke’s law of elasticity Force Extension = E × Area Length Hooke’s law σn = E εn where E is material constant, the Young’s Modulus Units are force/area – N/m2 or Pa Robert Hooke (1635-1703) was a virtuoso scientist contributing to geology, σ = C ε palaeontology, biology as well as mechanics ij ijkl kl ß Constitutive equations These are relationships between forces and deformation in a continuum, which define the material behaviour. GNH7/GG09/GEOL4002 EARTHQUAKE SEISMOLOGY AND EARTHQUAKE HAZARD Shear modulus and bulk modulus Young’s or stiffness modulus: σ n = Eε n Shear or rigidity modulus: σ S = Gε S = µε s Bulk modulus (1/compressibility): Mt Shasta andesite − P = Kεv Can write the bulk modulus in terms of the Lamé parameters λ, µ: K = λ + 2µ/3 and write Hooke’s law as: σ = (λ +2µ) ε GNH7/GG09/GEOL4002 EARTHQUAKE SEISMOLOGY AND EARTHQUAKE HAZARD Young’s Modulus or stiffness modulus Young’s Modulus or stiffness modulus: σ n = Eε n Interatomic force Interatomic distance GNH7/GG09/GEOL4002 EARTHQUAKE SEISMOLOGY AND EARTHQUAKE HAZARD Shear Modulus or rigidity modulus Shear modulus or stiffness modulus: σ s = Gε s Interatomic force Interatomic distance GNH7/GG09/GEOL4002 EARTHQUAKE SEISMOLOGY AND EARTHQUAKE HAZARD Hooke’s Law σij and εkl are second-rank tensors so Cijkl is a fourth-rank tensor. For a general, anisotropic material there are 21 independent elastic moduli. In the isotropic case this tensor reduces to just two independent elastic constants, λ and µ. -

Efficient Hydraulic Fluids Compressibility Is Still One of the Most Critically Important Factors
WORLDWIDE R. David Whitby Efficient hydraulic fluids Compressibility is still one of the most critically important factors. ydraulic fluids transmit power as a hydraulic system con- while phosphate and vegetable-oil esters have compressibilities H verts mechanical energy into fluid energy and subsequently similar to that of water. Polyalphaolefins are more compressible to mechanical work. To achieve this conversion efficiently, hydrau- than mineral oils. Water-glycol fluids are intermediate between lic fluids need to be relatively incompressible. Hydraulic fluids mineral oils and water. must also minimize wear, reduce friction, provide cooling and pre- Mineral oils are relatively incompressible, but volume reduc- vent rust and corrosion, be compatible with system components tions can be approximately 0.5% for pressures ranging from 6,900 and help keep the system free of deposits. kPa (1,000 psi) up to 27,600 kPa (4,000 psi). Compressibility in- Compressibility measures the rela- creases with pressure and temperature tive change in volume of a fluid or solid and has significant effects on high-pres- as a response to a change in pressure and sure fluid systems. Hydraulic oils typi- is the reciprocal of the volume-elastic cally contain 6% to 10% of dissolved air, modulus or bulk modulus of elasticity. which has no measurable effect on bulk Bulk modulus defines the pressure in- modulus provided it stays in solution. crease needed to cause a given relative Bulk modulus is an inherent property decrease in volume. of the hydraulic fluid and, therefore, is The bulk modulus of a fluid is nonlin- an inherent inefficiency of the hydraulic ear. -

Velocity, Density, Modulus of Hydrocarbon Fluids
Velocity, Density and Modulus of Hydrocarbon Fluids --Data Measurement De-hua Han*, HARC; M. Batzle, CSM Summary measured with pressure up to 55.2 Mpa (8000 Psi) and temperature up to 100 °C. Density and ultrasonic velocity of numerous hydrocarbon fluids (oil, oil based mud filtrate, hydrocarbon gases and Velocity of Dead Oil miscible CO2-oil) were measured at in situ conditions of pressure up to 50 Mpa and temperatures up to100 °C. Initial measurements were on the gas-free or ‘dead’ oils at Dynamic moduli are derived from velocities and densities. pressure and temperature (see Fig. 1). We used the Newly measured data refine correlations of velocity and following model to fit data: density to API gravity, Gas Oil ratio (GOR), Gas gravity and in situ pressure and temperature. Gas in solution is Vp (m/s) = A – B * T + C * P + D * T * P (1) largely responsible for reducing the bulk modulus of the live oil. Phase changes, such as exsolving gas during Here A is a pseudo velocity at 0 °C and room pressure (0 production, can dramatically lower velocities and modulus, Mpa, gauge), B is temperature gradient, C is pressure but is dependent on pressure conditions. Distinguish gas gradient and D is coefficient of coupled temperature and from liquid phase may not be possible at a high pressure. pressure effects. Fluids are often supercritical. With increasing pressure, a gas-like fluid can begin to behave like a liquid Dead & live oil of #1 B.P. = 1900 psi at 60 C Introduction 1800 1600 Hydrocarbon fluids are the primary targets of the seismic exploration. -

Lecture 42: Failure Analysis – Buckling of Columns Joshua Pribe Fall 2019 Lecture Book: Ch
ME 323 – Mechanics of Materials Lecture 42: Failure analysis – Buckling of columns Joshua Pribe Fall 2019 Lecture Book: Ch. 18 Stability and equilibrium What happens if we are in a state of unstable equilibrium? Stable Neutral Unstable 2 Buckling experiment There is a critical stress at which buckling occurs depending on the material and the geometry How do the material properties and geometric parameters influence the buckling stress? 3 Euler buckling equation Consider static equilibrium of the buckled pinned-pinned column 4 Euler buckling equation We have a differential equation for the deflection with BCs at the pins: d 2v EI+= Pv( x ) 0 v(0)== 0and v ( L ) 0 dx2 The solution is: P P A = 0 v(s x) = Aco x+ Bsin x with EI EI PP Bsin L= 0 L = n , n = 1, 2, 3, ... EI EI 5 Effect of boundary conditions Critical load and critical stress for buckling: EI EA P = 22= cr L2 2 e (Legr ) 2 E cr = 2 (Lreg) I r = Pinned- Pinned- Fixed- where g Fixed- A pinned fixed fixed is the “radius of gyration” free LLe = LLe = 0.7 LLe = 0.5 LLe = 2 6 Modifications to Euler buckling theory Euler buckling equation: works well for slender rods Needs to be modified for smaller “slenderness ratios” (where the critical stress for Euler buckling is at least half the yield strength) 7 Summary L 2 E Critical slenderness ratio: e = r 0.5 gYc Euler buckling (high slenderness ratio): LL 2 E EI If ee : = or P = 2 rr cr 2 cr L2 gg c (Lreg) e Johnson bucklingI (low slenderness ratio): r = 2 g Lr LLeeA ( eg) If : =−1 rr cr2 Y gg c 2 Lr ( eg)c with radius of gyration 8 Summary Effective length from the boundary conditions: Pinned- Pinned- Fixed- LL= LL= 0.7 FixedLL=- 0.5 pinned fixed e fixede e LL= 2 free e 9 Example 18.1 Determine the critical buckling load Pcr of a steel pipe column that has a length of L with a tubular cross section of inner radius ri and thickness t. -
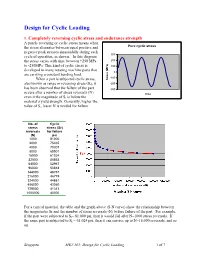
Design for Cyclic Loading, Soderberg, Goodman and Modified Goodman's Equation
Design for Cyclic Loading 1. Completely reversing cyclic stress and endurance strength A purely reversing or cyclic stress means when the stress alternates between equal positive and Pure cyclic stress negative peak stresses sinusoidally during each 300 cycle of operation, as shown. In this diagram the stress varies with time between +250 MPa 200 to -250MPa. This kind of cyclic stress is 100 developed in many rotating machine parts that 0 are carrying a constant bending load. -100 When a part is subjected cyclic stress, Stress (MPa) also known as range or reversing stress (Sr), it -200 has been observed that the failure of the part -300 occurs after a number of stress reversals (N) time even it the magnitude of Sr is below the material’s yield strength. Generally, higher the value of Sr, lesser N is needed for failure. No. of Cyclic stress stress (Sr) reversals for failure (N) psi 1000 81000 2000 75465 4000 70307 8000 65501 16000 61024 32000 56853 64000 52967 96000 50818 144000 48757 216000 46779 324000 44881 486000 43060 729000 41313 1000000 40000 For a typical material, the table and the graph above (S-N curve) show the relationship between the magnitudes Sr and the number of stress reversals (N) before failure of the part. For example, if the part were subjected to Sr= 81,000 psi, then it would fail after N=1000 stress reversals. If the same part is subjected to Sr = 61,024 psi, then it can survive up to N=16,000 reversals, and so on. Sengupta MET 301: Design for Cyclic Loading 1 of 7 It has been observed that for most of engineering materials, the rate of reduction of Sr becomes negligible near the vicinity of N = 106 and the slope of the S-N curve becomes more or less horizontal. -

Guide to Rheological Nomenclature: Measurements in Ceramic Particulate Systems
NfST Nisr National institute of Standards and Technology Technology Administration, U.S. Department of Commerce NIST Special Publication 946 Guide to Rheological Nomenclature: Measurements in Ceramic Particulate Systems Vincent A. Hackley and Chiara F. Ferraris rhe National Institute of Standards and Technology was established in 1988 by Congress to "assist industry in the development of technology . needed to improve product quality, to modernize manufacturing processes, to ensure product reliability . and to facilitate rapid commercialization ... of products based on new scientific discoveries." NIST, originally founded as the National Bureau of Standards in 1901, works to strengthen U.S. industry's competitiveness; advance science and engineering; and improve public health, safety, and the environment. One of the agency's basic functions is to develop, maintain, and retain custody of the national standards of measurement, and provide the means and methods for comparing standards used in science, engineering, manufacturing, commerce, industry, and education with the standards adopted or recognized by the Federal Government. As an agency of the U.S. Commerce Department's Technology Administration, NIST conducts basic and applied research in the physical sciences and engineering, and develops measurement techniques, test methods, standards, and related services. The Institute does generic and precompetitive work on new and advanced technologies. NIST's research facilities are located at Gaithersburg, MD 20899, and at Boulder, CO 80303. -

Multidisciplinary Design Project Engineering Dictionary Version 0.0.2
Multidisciplinary Design Project Engineering Dictionary Version 0.0.2 February 15, 2006 . DRAFT Cambridge-MIT Institute Multidisciplinary Design Project This Dictionary/Glossary of Engineering terms has been compiled to compliment the work developed as part of the Multi-disciplinary Design Project (MDP), which is a programme to develop teaching material and kits to aid the running of mechtronics projects in Universities and Schools. The project is being carried out with support from the Cambridge-MIT Institute undergraduate teaching programe. For more information about the project please visit the MDP website at http://www-mdp.eng.cam.ac.uk or contact Dr. Peter Long Prof. Alex Slocum Cambridge University Engineering Department Massachusetts Institute of Technology Trumpington Street, 77 Massachusetts Ave. Cambridge. Cambridge MA 02139-4307 CB2 1PZ. USA e-mail: [email protected] e-mail: [email protected] tel: +44 (0) 1223 332779 tel: +1 617 253 0012 For information about the CMI initiative please see Cambridge-MIT Institute website :- http://www.cambridge-mit.org CMI CMI, University of Cambridge Massachusetts Institute of Technology 10 Miller’s Yard, 77 Massachusetts Ave. Mill Lane, Cambridge MA 02139-4307 Cambridge. CB2 1RQ. USA tel: +44 (0) 1223 327207 tel. +1 617 253 7732 fax: +44 (0) 1223 765891 fax. +1 617 258 8539 . DRAFT 2 CMI-MDP Programme 1 Introduction This dictionary/glossary has not been developed as a definative work but as a useful reference book for engi- neering students to search when looking for the meaning of a word/phrase. It has been compiled from a number of existing glossaries together with a number of local additions. -

Making and Characterizing Negative Poisson's Ratio Materials
Making and characterizing negative Poisson’s ratio materials R. S. LAKES* and R. WITT**, University of Wisconsin–Madison, 147 Engineering Research Building, 1500 Engineering Drive, Madison, WI 53706-1687, USA. 〈[email protected]〉 Received 18th October 1999 Revised 26th August 2000 We present an introduction to the use of negative Poisson’s ratio materials to illustrate various aspects of mechanics of materials. Poisson’s ratio is defined as minus the ratio of transverse strain to longitudinal strain in simple tension. For most materials, Poisson’s ratio is close to 1/3. Negative Poisson’s ratios are counter-intuitive but permissible according to the theory of elasticity. Such materials can be prepared for classroom demonstrations, or made by students. Key words: Poisson’s ratio, deformation, foam, honeycomb 1. INTRODUCTION Poisson’s ratio is the ratio of lateral (transverse) contraction strain to longitudinal extension strain in a simple tension experiment. The allowable range of Poisson’s ratio ν in three 1 dimensional isotropic solids is from –1 to 2 [1]. Common materials usually have a Poisson’s 1 1 ratio close to 3 . Rubbery materials, however, have values approaching 2 . They readily undergo shear deformations, governed by the shear modulus G, but resist volumetric (bulk) deformation governed by the bulk modulus K; for rubbery materials G Ӷ K. Even though textbooks can still be found [2] which categorically state that Poisson’s ratios less than zero are unknown, or even impossible, there are in fact a number of examples of negative Poisson’s ratio solids. Although such behaviour is counter-intuitive, negative Poisson’s ratio structures and materials can be easily made and used in lecture demonstrations and for student projects. -

Hydraulics Manual Glossary G - 3
Glossary G - 1 GLOSSARY OF HIGHWAY-RELATED DRAINAGE TERMS (Reprinted from the 1999 edition of the American Association of State Highway and Transportation Officials Model Drainage Manual) G.1 Introduction This Glossary is divided into three parts: · Introduction, · Glossary, and · References. It is not intended that all the terms in this Glossary be rigorously accurate or complete. Realistically, this is impossible. Depending on the circumstance, a particular term may have several meanings; this can never change. The primary purpose of this Glossary is to define the terms found in the Highway Drainage Guidelines and Model Drainage Manual in a manner that makes them easier to interpret and understand. A lesser purpose is to provide a compendium of terms that will be useful for both the novice as well as the more experienced hydraulics engineer. This Glossary may also help those who are unfamiliar with highway drainage design to become more understanding and appreciative of this complex science as well as facilitate communication between the highway hydraulics engineer and others. Where readily available, the source of a definition has been referenced. For clarity or format purposes, cited definitions may have some additional verbiage contained in double brackets [ ]. Conversely, three “dots” (...) are used to indicate where some parts of a cited definition were eliminated. Also, as might be expected, different sources were found to use different hyphenation and terminology practices for the same words. Insignificant changes in this regard were made to some cited references and elsewhere to gain uniformity for the terms contained in this Glossary: as an example, “groundwater” vice “ground-water” or “ground water,” and “cross section area” vice “cross-sectional area.” Cited definitions were taken primarily from two sources: W.B. -

Determination of Shear Modulus of Dental Materials by Means of the Torsion Pendulum
NATIONAL BUREAU OF STANDARDS REPORT 10 068 Progress Report on DETERMINATION OF SHEAR MODULUS OF DENTAL MATERIALS BY MEANS OF THE TORSION PENDULUM U.S. DEPARTMENT OF COMMERCE NATIONAL BUREAU OF STANDARDS NATIONAL BUREAU OF STANDARDS The National Bureau of Standards ' was established by an act of Congress March 3, 1901. Today, in addition to serving as the Nation’s central measurement laboratory, the Bureau is a principal focal point in the Federal Government for assuring maximum application of the physical and engineering sciences to the advancement of technology in industry and commerce. To this end the Bureau conducts research and provides central national services in four broad program areas. These are: (1) basic measurements and standards, (2) materials measurements and standards, (3) technological measurements and standards, and (4) transfer of technology. The Bureau comprises the Institute for Basic Standards, the Institute for Materials Research, the Institute for Applied Technology, the Center for Radiation Research, the Center for Computer Sciences and Technology, and the Office for Information Programs. THE INSTITUTE FOR BASIC STANDARDS provides the central basis within the United States of a complete and consistent system of physical measurement; coordinates that system with measurement systems of other nations; and furnishes essential services leading to accurate and uniform physical measurements throughout the Nation’s scientific community, industry, and com- merce. The Institute consists of an Office of Measurement Services