2. Humphry Davy: Chemistry's First Showman
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Optical Timeline by Tony Oursler
Optical Timeline by Tony Oursler 1 Iris is thought to be derived from the RED Symyaz leads the fallen angels. Archimedes (c. 287212 b.c.) is said to Greek word for speaker or messenger. According to Enoch, they came to earth have used a large magnifying lens or Seth, the Egyptian god most associated of their own free will at Mount Hermon, burning-glass, which focused the suns Fifth century b.c. Chinese philosopher with evil, is depicted in many guises: descending like stars. This description rays, to set fire to Roman ships off Mo Ti, in the first description of the gives rise to the name Lucifer, “giver of Syracuse. camera obscura, refers to the pinhole as a black pig, a tall, double-headed figure light.” “collection place” and “locked treasure with a snout, and a serpent. Sometimes And now there is no longer any “I have seen Satan fall like lightning room.” he is black, a positive color for the difficulty in understanding the images in from heaven.” (Luke 10:1820) Egyptians, symbolic of the deep tones of mirrors and in all smooth and bright Platos Cave depicts the dilemma of fertile river deposits; at other times he is surfaces. The fires from within and from the uneducated in a graphic tableau of red, a negative color reflected by the without communicate about the smooth light and shadow. The shackled masses parched sands that encroach upon the surface, and from one image which is are kept in shadow, unable to move crops. Jeffrey Burton Russell suggests variously refracted. -

Mister Mary Somerville: Husband and Secretary
Open Research Online The Open University’s repository of research publications and other research outputs Mister Mary Somerville: Husband and Secretary Journal Item How to cite: Stenhouse, Brigitte (2020). Mister Mary Somerville: Husband and Secretary. The Mathematical Intelligencer (Early Access). For guidance on citations see FAQs. c 2020 The Author https://creativecommons.org/licenses/by/4.0/ Version: Version of Record Link(s) to article on publisher’s website: http://dx.doi.org/doi:10.1007/s00283-020-09998-6 Copyright and Moral Rights for the articles on this site are retained by the individual authors and/or other copyright owners. For more information on Open Research Online’s data policy on reuse of materials please consult the policies page. oro.open.ac.uk Mister Mary Somerville: Husband and Secretary BRIGITTE STENHOUSE ary Somerville’s life as a mathematician and mathematician). Although no scientific learned society had a savant in nineteenth-century Great Britain was formal statute barring women during Somerville’s lifetime, MM heavily influenced by her gender; as a woman, there was nonetheless a great reluctance even toallow women her access to the ideas and resources developed and into the buildings, never mind to endow them with the rights circulated in universities and scientific societies was highly of members. Except for the visit of the prolific author Margaret restricted. However, her engagement with learned institu- Cavendish in 1667, the Royal Society of London did not invite tions was by no means nonexistent, and although she was women into their hallowed halls until 1876, with the com- 90 before being elected a full member of any society mencement of their second conversazione [15, 163], which (Societa` Geografica Italiana, 1870), Somerville (Figure 1) women were permitted to attend.1 As late as 1886, on the nevertheless benefited from the resources and social nomination of Isis Pogson as a fellow, the Council of the Royal networks cultivated by such institutions from as early as Astronomical Society chose to interpret their constitution as 1812. -

Humphry Davy: Science, Authorship, and the Changing Romantic
Brigham Young University BYU ScholarsArchive Theses and Dissertations 2010-11-29 Humphry Davy: Science, Authorship, and the Changing Romantic Marianne Lind Baker Brigham Young University - Provo Follow this and additional works at: https://scholarsarchive.byu.edu/etd Part of the English Language and Literature Commons BYU ScholarsArchive Citation Baker, Marianne Lind, "Humphry Davy: Science, Authorship, and the Changing Romantic" (2010). Theses and Dissertations. 2647. https://scholarsarchive.byu.edu/etd/2647 This Thesis is brought to you for free and open access by BYU ScholarsArchive. It has been accepted for inclusion in Theses and Dissertations by an authorized administrator of BYU ScholarsArchive. For more information, please contact [email protected], [email protected]. Title Page Humphry Davy: Science, Authorship, and the Changing ―Romantic I‖ Marianne Lind Baker A thesis submitted to the faculty of Brigham Young University in partial fulfillment of the requirements for the degree of Master of Arts Nicholas Mason, Chair Leslee Thorne-Murphy Paul Westover Department of English Brigham Young University December 2010 Copyright © 2010 Marianne Baker All Rights Reserved Abstract ABSTRACT Humphry Davy: Science, Authorship, and the Changing ―Romantic I‖ Marianne Lind Baker Department of English Master of Arts In the mid to late 1700s, men of letters became more and more interested in the natural world. From studies in astronomy to biology, chemistry, and medicine, these ―philosophers‖ pioneered what would become our current scientific categories. While the significance of their contributions to these fields has been widely appreciated historically, the interconnection between these men and their literary counterparts has not. A study of the ―Romantic man of science‖ reveals how much that figure has in common with the traditional ―Romantic‖ literary figure embodied by poets like William Wordsworth and Samuel Taylor Coleridge. -

RIDDLE and MYSTERY a Tapestry of Faith Program for Children 6Th Grade
RIDDLE AND MYSTERY A Tapestry of Faith Program for Children 6th Grade BY RICHARD S. KIMBALL © Copyright 2010 Unitarian Universalist Association. This program and additional resources are available on the UUA.org web site at www.uua.org/re/tapestry 1 TABLE OF CONTENTS ABOUT THE AUTHORS ......................................................................................................................................................................... 3 ACKNOWLEDGMENTS ......................................................................................................................................................................... 3 THE PROGRAM ....................................................................................................................................................................................... 4 SESSION 1: THE BIG QUESTIONS ..................................................................................................................................................... 15 SESSION 2: RELIGION TO THE RESCUE .......................................................................................................................................... 35 SESSION 3: LOOKING TOWARD TOMORROW ............................................................................................................................... 54 SESSION 4: THINKING OF GOD ......................................................................................................................................................... 74 SESSION -

Proceedings and Index of the 68Th Annual Convention
Proceedings and Index of the 68th Annual Convention Communications Workers of America Las Vegas Hilton Hotel Las Vegas, Nevada July 10-11, 2006 MONDAY MORNING SESSION July 10, 2006 The Opening Session of the Sixty-Eighth Annual Convention of the Communications Workers of America, held in the Conrad Ballroom of the Las Vegas Hilton Hotel and Casino, Las Vegas, Nevada, July 10-11, 2006, convened at 9:00 a.m., Louie Rocha, President, CWA Local 9423, Chair of the Northern California/Nevada Council, Temporary Chair, presiding. TEMPORARY CHAIR ROCHA: Good Morning! Buenos dias! We have a very full agenda this morning, so I ask all of you to take your seats. Will all of the delegates please take their seats. The official clock of the Northern California Council indicates that it is now 9:00 a.m. I would ask that everyone please be seated as I call the 68th Annual Convention of the Communications Workers of America to order. We have a busy schedule this morning and a very full two-day schedule, so would everyone please be seated so we can begin. I am Louie Rocha, President of the Northern California and Nevada Council and President of CWA Local 9423. Today I have the honor of serving as the Temporary Chair. On behalf of the officers and members of District 9, welcome to Las Vegas and the 68th Annual Convention of the Communications Workers of America. (Applause) As is our custom, we will open our Convention with a prayer. For the invocation, I would like to call upon Reverend Peter Romeo, Judicial Vicar of the Diocese of Las Vegas, Nevada. -
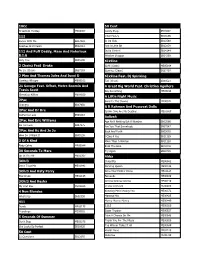
Karaoke Song List
10Cc 50 Cent Dreadlock Holiday MX00002 Candy Shop SB13602 112 I Got Money SB16596 Dance With Me SB17659 In Da Club SB12588 Peaches And Cream SB12012 Just A Little Bit SB13839 112 And Puff Daddy, Mase And Notorious Outta Control SB14144 B.I.G Window Shopper SB14269 Only You SB05190 6Ix9ine 2 Chainz Feat Drake Gotti (Clean) ME05164 No Lie (Clean) SB27103 Gummo (Clean) SB32797 2 Play And Thomas Jules And Jucxi D 6Ix9ine Feat. Dj Spinking Careless Whisper ME00102 Tati (Clean) SB33521 21 Savage Feat. Offset, Metro Boomin And A Great Big World Feat. Christina Aguilera Travis Scott Say Something ME03194 Ghostface Killers ME04019 A Little Night Music 2Pac Send In The Clowns ME00572 Changes SB07950 A R Rahman And Pussycat Dolls 2Pac And Dr Dre Jai Ho (You Are My Destiny) ME01867 California Love SB04883 Aaliyah 2Pac And Eric Williams Age Ain't Nothing But A Number SB03566 Do For Love SB07275 Are You That Somebody SB07547 2Pac And Kc And Jo Jo Back And Forth SB03062 How Do U Want It SB05238 I Care 4 You SB11129 3 Of A Kind More Than A Woman SB11128 Baby Cakes ME00044 Rock The Boat SB12016 30 Seconds To Mars Try Again SB09709 Up In The Air ME02907 Abba 3Oh!3 Chiquitita ME00982 Don't Trust Me ME01942 Dancing Queen ME00146 3Oh!3 And Katy Perry Does Your Mother Know ME01124 Starstrukk ME02145 Fernando ME00989 3Oh!3 And Kesha Gimme Gimme Gimme ME00219 My First Kiss ME02263 I Have A Dream ME00288 4 Non Blondes Knowing Me Knowing You ME00372 What's Up SB02550 Mamma Mia ME00425 411 Money Money Money ME00449 Dumb ME00175 S.O.S ME00560 Teardrops ME00651 Super -

Steel Production Through Electrolysis: Impacts for Electricity Consumption 0, 0, 75
Font Family: Benton Sans 131, 176, 70 Steel production through electrolysis: impacts for electricity consumption 0, 0, 75 204, 102, 51 Adam Rauwerdink 144, 144, 144 VP, Business Development October 18, 2019 Font Family: A 3,000 year old formula Benton Sans Iron Ore Carbon (Coal) Iron Carbon Dioxide 131, 176, 70 Fe2O3 C Fe CO2 0, 0, 75 204, 102, 51 >2 144, 144, 144 Gt CO2 per year (8% of global emissions) Iron Age 1000 BC Digital Age 2019 2 Boston Metal | 2019 Font Family: Steel in 2018 Benton Sans 131, 176, 70 Aluminium is #2 at 1,800 64 million tonnes 0, 0, 75 million tonnes 204, 102, 51 70% 30% 144, 144, 144 Integrated Steel Mill Mini Mill (Iron ore new steel units) (Scrap recycled steel units) Source: World Steel Association 3 Boston Metal | 2019 Font Family: Integrated steel mill: material flow Benton Sans 131, 176, 70 0, 0, 75 204, 102, 51 144, 144, 144 4 Boston Metal | 2019 Font Family: Molten oxide electrolysis (MOE) is emissions free Benton Sans Molten Oxide Electrolysis (MOE) 131, 176, 70 Iron Ore Electricity Iron Oxygen 0, 0, 75 - Fe2O3 e Fe O2 204, 102, 51 144, 144, 144 No carbon in the process = No CO2 emitted Electricity decarbonization eliminates/reduces indirect emissions! 5 Boston Metal | 2019 Font Family: Changing the formula from coal to electricity Benton Sans Iron Ore Carbon (Coal) Iron Carbon Dioxide 131, 176, 70 Fe2O3 C Fe CO2 0, 0, 75 204, 102, 51 Molten Oxide Electrolysis (MOE) 144, 144, 144 Iron Ore Electricity Iron Oxygen - Fe2O3 e Fe O2 6 Boston Metal | 2019 Font Family: MOE is more energy efficient Benton -

The Early History of Catalysis
The Early History of Catalysis By Professor A. J. B. Robertson Department of Chemistry, King’s College, London One hundred and forty years ago it was Berzelius proceeded to propose the exist- possible for one man to prepare an annual ence of a new force which he called the report on the progress of the whole of “catalytic force” and he called “catalysis” the chemistry, and for many years this task was decomposition of bodies by this force. This undertaken by the noted Swedish chemist is probably the first recognition of catalysis J. J. Berzelius for the Stockholm Academy of as a wide-ranging natural phenomenon. Sciences. In his report submitted in 1835 and Metallic catalysts had in fact been used in published in 1836 Berzelius reviewed a num- the laboratory before 1800 by Joseph Priestley, ber of earlier findings on chemical change in the discoverer of oxygen, and by the Dutch both homogeneous and heterogeneous sys- chemist Martinus van Marum, both of whom tems, and showed that these findings could be made observations on the dehydrogenation of rationally co-ordinated by the introduction alcohol on metal catalysts. However, it seems of the concept of catalysis. In a short paper likely that these investigators regarded the summarising his ideas on catalysis as a new metal merely as a source of heat. In 1813, force, he wrote (I): Louis Jacques Thenard discovered that ammonia is decomposed into nitrogen and “It is, then, proved that several simple or compound bodies, soluble and insoluble, have hydrogen when passed over various red-hot the property of exercising on other bodies an metals, and ten years later, with Pierre action very different from chemical affinity. -

Electrochemistry of Fuel Cell - Kouichi Takizawa
ENERGY CARRIERS AND CONVERSION SYSTEMS – Vol. II - Electrochemistry of Fuel Cell - Kouichi Takizawa ELECTROCHEMISTRY OF FUEL CELL Kouichi Takizawa Tokyo Electric Power Company, Tokyo, Japan Keywords : electrochemistry, fuel cell, electrochemical reaction, chemical energy, anode, cathode, electrolyte, Nernst equation, hydrogen-oxygen fuel cell, electromotive force Contents 1. Introduction 2. Principle of Electricity Generation by Fuel Cells 3. Electricity Generation Characteristics of Fuel Cells 4. Fuel Cell Efficiency Glossary Bibliography Biographical Sketch Summary Fuel cells are devices that utilize electrochemical reactions to generate electric power. They are believed to give a significant impact on the future energy system. In particular, when hydrogen can be generated from renewable energy resources, it is certain that the fuel cell should play a significant role. Even today, some types of fuel cells have been already used in practical applications such as combined heat and power generation applications and space vehicle applications. Though research and development activities are still required, the fuel cell technology is one of the most important technologies that allow us to draw the environment friendly society in the twenty-first century. This section describes the general introduction of fuel cell technology with a brief overview of the principle of fuel cells and their historical background. 1. Introduction A fuel cellUNESCO is a system of electric power – generation,EOLSS which utilizes electrochemical reactions. It can produce electric power by inducing both a reaction to oxidize hydrogen obtained by reforming natural gas or other fuels, and a reaction to reduce oxygen in the air, each occurringSAMPLE at separate electrodes conne CHAPTERScted to an external circuit. -

Teaching H. C. Ørsted's Scientific Work in Danish High School Physics
UNIVERSITY OF COPENHAGEN FACULTY OF SCIENCE Ida Marie Monberg Hindsholm Teaching H. C. Ørsted's Scientific Work in Danish High School Physics Masterʹs thesis Department of Science Education 19 July 2018 Master's thesis Teaching H. C. Ørsted’s Scientific Work in Danish High School Physics Submitted 19 July 2018 Author Ida Marie Monberg Hindsholm, B.Sc. E-mail [email protected] Departments Niels Bohr Institute, University of Copenhagen Department of Science Education, University of Copenhagen Main supervisor Ricardo Avelar Sotomaior Karam, Associate Professor, Department of Science Education, University of Copenhagen Co-supervisor Steen Harle Hansen, Associate Professor, Niels Bohr Institute, University of Copenhagen 1 Contents 1 Introduction . 1 2 The Material: H. C. Ørsted's Work . 3 2.1 The Life of Hans Christian Ørsted . 3 2.2 Ørsted’s Metaphysical Framework: The Dynamical Sys- tem............................. 6 2.3 Ritter and the failure in Paris . 9 2.4 Ørsted’s work with acoustic and electric figures . 12 2.5 The discovery of electromagnetism . 16 2.6 What I Use for the Teaching Sequence . 19 3 Didactic Theory . 20 3.1 Constructivist teaching . 20 3.2 Inquiry Teaching . 22 3.3 HIPST . 24 4 The Purpose and Design of the Teaching Sequence . 27 4.1 Factual details and lesson plan . 28 5 Analysis of Transcripts and Writings . 40 5.1 Method of Analysis . 40 5.2 Practical Problems . 41 5.3 Reading Original Ørsted's Texts . 42 5.4 Inquiry and Experiments . 43 5.5 "Role play" - Thinking like Ørsted . 48 5.6 The Reflection Corner . 51 5.7 Evaluation: The Learning Objectives . -

5. the Lives of Two Pioneering Medical Chemists.Indd
The West of England Medical Journal Vol 116 No 4 Article 2 Bristol Medico-Historical Society Proceedings The Lives of Two Pioneering Medical-Chemists in Bristol Thomas Beddoes (1760-1808) and William Herapath (1796-1868) Brian Vincent School of Chemistry, University of Bristol, Bristol, BS8 1TS. Presented at the meeting on Dec 12th 2016 ABSTRACT From the second half of the 18th century onwards the new science of chemistry took root and applications were heralded in many medical-related fields, e.g. cures for diseases such as TB, the prevention of epidemics like cholera, the application of anaesthetics and the detection of poisons in forensics. Two pioneering chemists who worked in the city were Thomas Beddoes, who founded the Pneumatic Institution in Hotwells in 1793, and William Herapath who was the first professor of chemistry and toxicology at the Bristol Medical School, located near the Infirmary, which opened in 1828. As well as their major contributions to medical-chemistry, both men played important roles in the political life of the city. INTRODUCTION The second half of the 18th century saw chemistry emerge as a fledgling science. Up till then there was little understanding of the true nature of matter. The 1 The West of England Medical Journal Vol 116 No 4 Article 2 Bristol Medico-Historical Society Proceedings classical Greek idea that matter consisted of four basic elements (earth, fire, water and air) still held sway, as did the practice of alchemy: the search for the “elixir of life” and for the “philosophers’ stone” which would turn base metals into gold. -

Electrochemistry –An Oxidizing Agent Is a Species That Oxidizes Another Species; It Is Itself Reduced
Oxidation-Reduction Reactions Chapter 17 • Describing Oxidation-Reduction Reactions Electrochemistry –An oxidizing agent is a species that oxidizes another species; it is itself reduced. –A reducing agent is a species that reduces another species; it is itself oxidized. Loss of 2 e-1 oxidation reducing agent +2 +2 Fe( s) + Cu (aq) → Fe (aq) + Cu( s) oxidizing agent Gain of 2 e-1 reduction Skeleton Oxidation-Reduction Equations Electrochemistry ! Identify what species is being oxidized (this will be the “reducing agent”) ! Identify what species is being •The study of the interchange of reduced (this will be the “oxidizing agent”) chemical and electrical energy. ! What species result from the oxidation and reduction? ! Does the reaction occur in acidic or basic solution? 2+ - 3+ 2+ Fe (aq) + MnO4 (aq) 6 Fe (aq) + Mn (aq) Steps in Balancing Oxidation-Reduction Review of Terms Equations in Acidic solutions 1. Assign oxidation numbers to • oxidation-reduction (redox) each atom so that you know reaction: involves a transfer of what is oxidized and what is electrons from the reducing agent to reduced 2. Split the skeleton equation into the oxidizing agent. two half-reactions-one for the oxidation reaction (element • oxidation: loss of electrons increases in oxidation number) and one for the reduction (element decreases in oxidation • reduction: gain of electrons number) 2+ 3+ - 2+ Fe (aq) º Fe (aq) MnO4 (aq) º Mn (aq) 1 3. Complete and balance each half reaction Galvanic Cell a. Balance all atoms except O and H 2+ 3+ - 2+ (Voltaic Cell) Fe (aq) º Fe (aq) MnO4 (aq) º Mn (aq) b.