Checchi, Arthur A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Summer Camps, As Camps Will Be Cancelled for Low Enrollment
BENICIA PARKS & COMMUNITY SERVICES DEPARTMENT CONTACTS Mike Dotson, Director Ann Dunleavy, Superintendent OFFICE LOCATION: Benicia Community Center Theron Jones, Superintendent 370 East L Street Victor Randall, Management Analyst Benicia, CA 94510 Lindsay Zarcone, Recreation Supervisor Phone: (707) 746-4285 Wendy Stratton Monahan, Recreation Supervisor www.beniciarec.org Eliot Palmer, Recreation Supervisor [email protected] Debbi Bray, Administrative Clerk Rachel Morgado, Recreation Specialist OFFICE HOURS: Monday–Friday, 8am – 5pm For direct contact information, visit www.beniciarec.org You can register for our classes and programs at our office, or online at www.beniciarec. org. Payments at the office can be made with cash, check, VISA, Mastercard, or American Express. Online registrations requires a login and password. Please stop by or call our office to set up an account to access online registration. Full credit will be given to cancellations received 72 hours prior to the first class, unless otherwise noted in the program description. Failure to attend a class session will not be granted a credit/refund. Trip refunds will only be considered with prior notice of 15 days or more before trip departure. Exceptions are at the discretion of the program supervisor. Time, dates, and locations of classes are subject to change. Please contact the Recreation Supervisor if you are not satisfied with a program. We offer a satisfaction guaranteed policy. Credits/refunds will be offered if requested prior to the second class session, if you are not satisfied with the program. This guarantee does not apply to youth and adult sports leagues, trips, facility rentals, recreation swim, and single day events, classes, or activities. -

Identifying Handmade and Machine Lace Identification
Identifying Handmade and Machine Lace DATS in partnership with the V&A DATS DRESS AND TEXTILE SPECIALISTS 1 Identifying Handmade and Machine Lace Text copyright © Jeremy Farrell, 2007 Image copyrights as specified in each section. This information pack has been produced to accompany a one-day workshop of the same name held at The Museum of Costume and Textiles, Nottingham on 21st February 2008. The workshop is one of three produced in collaboration between DATS and the V&A, funded by the Renaissance Subject Specialist Network Implementation Grant Programme, administered by the MLA. The purpose of the workshops is to enable participants to improve the documentation and interpretation of collections and make them accessible to the widest audiences. Participants will have the chance to study objects at first hand to help increase their confidence in identifying textile materials and techniques. This information pack is intended as a means of sharing the knowledge communicated in the workshops with colleagues and the public. Other workshops / information packs in the series: Identifying Textile Types and Weaves 1750 -1950 Identifying Printed Textiles in Dress 1740-1890 Front cover image: Detail of a triangular shawl of white cotton Pusher lace made by William Vickers of Nottingham, 1870. The Pusher machine cannot put in the outline which has to be put in by hand or by embroidering machine. The outline here was put in by hand by a woman in Youlgreave, Derbyshire. (NCM 1912-13 © Nottingham City Museums) 2 Identifying Handmade and Machine Lace Contents Page 1. List of illustrations 1 2. Introduction 3 3. The main types of hand and machine lace 5 4. -

IPG Spring 2020 Rock Pop and Jazz Titles
Rock, Pop, and Jazz Titles Spring 2020 {IPG} That Thin, Wild Mercury Sound Dylan, Nashville, and the Making of Blonde on Blonde Daryl Sanders Summary That Thin, Wild Mercury Sound is the definitive treatment of Bob Dylan’s magnum opus, Blonde on Blonde , not only providing the most extensive account of the sessions that produced the trailblazing album, but also setting the record straight on much of the misinformation that has surrounded the story of how the masterpiece came to be made. Including many new details and eyewitness accounts never before published, as well as keen insight into the Nashville cats who helped Dylan reach rare artistic heights, it explores the lasting impact of rock’s first double album. Based on exhaustive research and in-depth interviews with the producer, the session musicians, studio personnel, management personnel, and others, Daryl Sanders Chicago Review Press chronicles the road that took Dylan from New York to Nashville in search of “that thin, wild mercury sound.” 9781641602730 As Dylan told Playboy in 1978, the closest he ever came to capturing that sound was during the Blonde on Pub Date: 5/5/20 On Sale Date: 5/5/20 Blonde sessions, where the voice of a generation was backed by musicians of the highest order. $18.99 USD Discount Code: LON Contributor Bio Trade Paperback Daryl Sanders is a music journalist who has worked for music publications covering Nashville since 1976, 256 Pages including Hank , the Metro, Bone and the Nashville Musician . He has written about music for the Tennessean , 15 B&W Photos Insert Nashville Scene , City Paper (Nashville), and the East Nashvillian . -
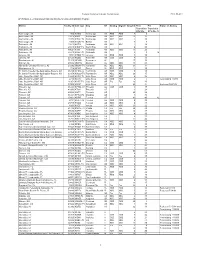
APPENDIX a – Initial List of Stations Eligible for Analog Nightlight Program
Federal Communications Commission FCC 08-281 APPENDIX A – Initial List of Stations Eligible for Analog Nightlight Program Market Facility ID Call sign City ST Analog Digital Anlg Ch. Post Pre Status of Analog Transition Transition DTV Ch. DTV Ch. (*) Anchorage, AK 804 KAKM Anchorage AK PBS PBS 7 8 Anchorage, AK 13815 KIMO Anchorage AK ABC ABC 13 12 Anchorage, AK 10173 KTUU-TV Anchorage AK NBC NBC 2 10 Anchorage, AK 4983 KYUK-TV Bethel AK 4 3 Fairbanks, AK 13813 KATN Fairbanks AK ABC ABC 2 18 Fairbanks, AK 20015 KJNP-TV North Pole AK 4 20 Fairbanks, AK 49621 KTVF Fairbanks AK NBC NBC 11 26 Fairbanks, AK 69315 KUAC-TV Fairbanks AK 9 9 24 Juneau, AK 8651 KTOO-TV Juneau AK PBS PBS 3 10 Juneau, AK 60520 KUBD Ketchikan AK CBS CBS 4 13 Birmingham, AL 71325 WDBB Bessemer AL 17 18 Dothan, AL 43846 WDHN Dothan AL ABC ABC 18 21 Huntsville-Decatur-Florence, AL 57292 WAAY-TV Huntsville AL ABC ABC 31 32 Montgomery, AL 714 WDIQ Dozier AL PBS PBS 2 10 Ft. Smith-Fayetteville-Springdale-Rogers, AR 66469 KFSM-TV Fort Smith AR CBS CBS 5 18 Ft. Smith-Fayetteville-Springdale-Rogers, AR 60354 KHOG-TV Fayetteville AR ABC ABC 29 15 Little Rock-Pine Bluff, AR 33440 KARK-TV Little Rock AR NBC NBC 4 32 Little Rock-Pine Bluff, AR 2770 KETS Little Rock AR PBS PBS 2 7 Terminating 1/3/09 Little Rock-Pine Bluff, AR 11951 KLRT-TV Little Rock AR Fox Fox 16 30 Little Rock-Pine Bluff, AR 37005 KWBF Little Rock AR 42 44 Reduced 10/31/08 Phoenix, AZ 41223 KPHO-TV Phoenix AZ CBS CBS 5 17 Phoenix, AZ 40993 KTVK Phoenix AZ 3 24 Phoenix, AZ 68886 KUTP Phoenix AZ 45 26 Tucson, -

Hooray for Health Arthur Curriculum
Reviewed by the American Academy of Pediatrics HHoooorraayy ffoorr HHeeaalltthh!! Open Wide! Head Lice Advice Eat Well. Stay Fit. Dealing with Feelings All About Asthma A Health Curriculum for Children IS PR O V IDE D B Y FUN D ING F O R ARTHUR Dear Educator: Libby’s® Juicy Juice® has been a proud sponsor of the award-winning PBS series ARTHUR® since its debut in 1996. Like ARTHUR, Libby’s Juicy Juice, premium 100% juice, is wholesome and loved by kids. Promoting good health has always been a priority for us and Juicy Juice can be a healthy part of any child’s balanced diet. Because we share the same commitment to helping children develop and maintain healthy lives, we applaud the efforts of PBS in producing quality educational television. Libby’s Juicy Juice hopes this health curriculum will be a valuable resource for teaching children how to eat well and stay healthy. Enjoy! Libby’s Juicy Juice ARTHUR Health Curriculum Contents Eat Well. Stay Fit.. 2 Open Wide! . 7 Dealing with Feelings . 12 Head Lice Advice . 17 All About Asthma . 22 Classroom Reproducibles. 30 Taping ARTHUR™ Shows . 32 ARTHUR Home Videos. 32 ARTHUR on the Web . 32 About This Guide Hooray for Health! is a health curriculum activity guide designed for teachers, after-school providers, and school nurses. It was developed by a team of health experts and early childhood educators. ARTHUR characters introduce five units exploring five distinct early childhood health themes: good nutrition and exercise (Eat Well. Stay Fit.), dental health (Open Wide!), emotions (Dealing with Feelings), head lice (Head Lice Advice), and asthma (All About Asthma). -

Alcohol and Alcohol Safetyi a Curriculummanqal for Senior High Level
pocoiENT -RESUME CG,011 580' ED.1.41 733 - AUTHOR Finn, Peter; Platt,,Judith-' , TITLE .Alcohol and Alcohol Safetyi A CurriculumManqal for Senior High Level. Volume II,A Teacher's Acfi..yities .. / . Guide. INSTITUTION Abt AsSociates, Inc.Cambiidge, Mass. SPO_RS AGENCY National Highway Traffic SafetyAdministration (Dq!, :Washington, D. C.; National Inst. onAlcohol'Abuse and Alcoholism (DHEW/PHS), Rockville, Md. - REPORT NO DOT-HS-800-70E 'PUB DATE Sep 72 . CONTRACT HSM-42-71-77 --- NOTE 500p.; For .Volume I, see ED672 383 AVAILABLE FROM Superintendent of,Documents, U.S. Government Printing Office, Washingto.C.. 20402 , EDRS PRICE ". MF-7$1-.00 HC-$26.11 Plus Postage. DESCRIPTORS *Alcohol Education; Alcoholism; *Drinking; Instructional Materials;-Interpersonal Competence; , , Laws; Lesson Tlans;.SecondaryEducation; *Senior High Schools; *Social *Attitudes.; *Social Behavior; Teaching Guides. a ABSTRACT ma3/41ual 'on Alcohol, and Alcohol Saf'ety . This curriculum is designed, .as ateacher's guidefor seniot high, level students. The topics it covers are,:(1) safety;.(2) attitudes towardalcohol'gnd reasons people drink.; (3) physical and behavioral effects.; (4) ' alcohol industty;(5) interpersonalsituations;(6) laws and customs;.. and (7),problem drinking andalcoholism. Each topic is divided into a number of activities. Each activityis,a self-contained learning -experience which requires varying numbersof class periods, and: focuses On one or More objectives.The particulaf skills developed by'' the &ctivity, as well as methods forevaluating it, are provided. ' ACtivities are also organized by teaChingmethod: art, audiovisual, debates., discussion, drama, independentstudy, 4ctures, 'reading, science"and, writing. (BP) . * * Documents 'acquired byERIC.incrude many informal unpublished * materials not available, fromother sources. ERIC makes'everY effort * * to obtain the best copyavailable. -

Kids with Asthma Can! an ACTIVITY BOOKLET for PARENTS and KIDS
Kids with Asthma Can! AN ACTIVITY BOOKLET FOR PARENTS AND KIDS Kids with asthma can be healthy and active, just like me! Look inside for a story, activity, and tips. Funding for this booklet provided by MUSEUMS, LIBRARIES AND PUBLIC BROADCASTERS JOINING FORCES, CREATING VALUE A Corporation for Public Broadcasting and Institute of Museum and Library Services leadership initiative PRESENTED BY Dear Parents and Friends, These days, almost everybody knows a child who has asthma. On the PBS television show ARTHUR, even Arthur knows someone with asthma. It’s his best friend Buster! We are committed to helping Boston families get the asthma care they need. More and more children in Boston these days have asthma. For many reasons, children in cities are at extra risk of asthma problems. The good news is that it can be kept under control. And when that happens, children with asthma can do all the things they like to do. It just takes good asthma management. This means being under a doctor’s care and taking daily medicine to prevent asthma Watch symptoms from starting. Children with asthma can also take ARTHUR ® quick relief medicine when asthma symptoms begin. on PBS KIDS Staying active to build strong lungs is a part of good asthma GO! management. Avoiding dust, tobacco smoke, car fumes, and other things that can start an asthma attack is important too. We hope this booklet can help the children you love stay active with asthma. Sincerely, 2 Buster’s Breathless Adapted from the A RTHUR PBS Series A Read-Aloud uster and Arthur are in the tree house, reading some Story for B dusty old joke books they found in Arthur’s basement. -

Fabled King Arthur 'Was Buried on Iona'
10 News Talk to us w scotlandonsunday.com myscotlandonsunday @scotonsunday November 17,2013 Fabled King Arthur ‘was buried on Iona’ Historian’sbooksayslegendary courtofCamelot wasinArgyll Emma Cowing says thattheycan be proved “beyond reasonable doubt”. WELCOME to MacCamelot. Theassertions in his book King Arthur was aScottish, Finding Arthur: The True pre-Christian warlord whose Origins Of The Once And remains are buried on Iona, FutureKing are strengthened according to anew book by a by the discoveryin2011of Scots historian. whatsome experts believe is Author Adam Ardreyclaims King Arthur’s round table in thatinstead of the romantic the grounds of Stirling Castle. English king of legend who Ardreysayshenot only be- lived at Camelot –which is lieves Arthur is buried in Iona often said to be Tintagel in but would love to see the site Cornwall or in Wales –Arthur excavated to look for proof. was actually Arthur Mac “The legendaryArthur is » Arthur is said to have pulled Excalibur from astone at Dunadd and, right, Aedan, the sixth-centuryson said to be buried in an island Iona Abbeyonthe isle whereitisclaimed he wasburied. Photograph: Alamy of an ancient King of Scotland, in the western seas –Avalon – whose Camelot was amarsh in but in the south of Britain of connectionsbetween Argyll land, including Stow in the cally,aswell as historically Thelegend of Arthur devel- It was hoped thatthe discov- Thebook also asserts that Argyll. there are no islands in the and Arthur are so numerous Borders, where he says the place them in context. Six of oped in the Middle Ages large- erycould prove thatKing Arthur became the victim of He also suggeststhatArthur western seas,”hesays. -

Asthma Education Handout
Asthma and Your Airways during an Normal Asthmatic Asthmatic attack airway airway airway Air trapped Tightened in alveoli smooth muscles Relaxed smooth muscles Wall inflamed and thickened Inhaled Corticosteroid Albuterol Salmeterol Singulair Levalbuterol Formoterol Oral Steroid Ipatropium Bromide Intra venous Steroid Types of Therapy Daily or Preventive – Used everyday and may be increased when sick 1. Inhaled Corticosteroid – Small amount of steroid inhaled into the airway. Decrease swelling and limit side effects from taking intravenous or oral steroids + Mometazone + Budesonide + Ciclesonide + Fluticasone + Beclomethasone 2. Long Acting Bronchodialators – Relax airway muscles that decrease airway size + Salmeterol + Formoterol 3. Leukotriene Receptor Antagonist– Decrease swelling in the airway + Montelukast Acute or Rescue Medicines – These are only used when asthma symptoms are present Short Acting Bronchodialators - Relax airway muscles that squeeze small airways during acute asthma attacks + Albuterol + Levalbuterol + Ipratropium Bromide What Causes or Triggers Asthma? Respiratory Illness (most common) + colds + sore throats + flu (influenza) + sinus infections + pneumonia Allergies (Allergic Asthma) + dust mites + molds + cockroach + pet dander + pollens + rodents Irritants in the Air + smoke from cigarettes + strong fumes, vapors, or odors + air pollution + dusts and particles in the air + wood or charcoal fires + chemicals Feeling and Expressing Strong Emotions + anger + laughter + fear + yelling + excitement + crying Exercise -

THE TICK (A Parody) by Ben Edlund the TICK a Parody
THE TICK (A Parody) by Ben Edlund THE TICK A Parody FADE IN: EXT. EARTH - DAY The screen is filled with a perfect TICK BLUE. The whistling growl of A LOW TUVAN SINGER HUM intones. WIDEN SLOWLY TO REVEAL it’s the blue of an ocean from orbit. TICK (V.O.) Hey! HUMANITY! WIDENER - EARTH hangs in space. Just visible past it, the SUN scatters glancing rays in a dazzle of beauty. MORE VOICES join the deep HUM, build to a ‘Zarathustra’ chorus. TICK (V.O.) Long has been your adventure! Blood and broad-swords and dynasty and ziggurats and SO MUCH shipping! Man, you cats love movin’ stuff around... (deep seriousness) And always, underneath it all, the Eternal Struggle. A BLACK SHAPE tumbles over us: A MASSIVE ASTEROID falling towards our world. TICK (V.O.) The story of Light against Darkness. Of Good against Evil. The Battle for the fate of the world... The Hero’s Journey! Earth’s bottom arc fills upper frame. The tiny ASTEROID rises up vertically to it, trailing a WHITE HOT TAIL OF MOLTEN STRATOSPHERE [echo of sperm and ovum? YES!]. TICK (V.O.) This then, is that story. The most important story ever told... The blazing asteroid falls at us, FILLING FRAME with FIRE. A title SUPERS over the ROILING INCANDESCENCE: “THE TICK” TICK (V.O.) My story. 2/3/16 TICK 'Pilot' 2 EXT. TUNGUSKA RIVER BASIN - DAY Gorgeous vista. Trackless arctic forest. Dawn. The ASTEROID descends from the dome of sky. The end is near. ON REINDEER HERD - which chuffs and steams, clearing frame to reveal a TWO EVENKI TRIBESMEN; fur-clad, indigenous Siberians. -

Arthur WN Guide Pdfs.8/25
Building Global and Cultural Awareness Keep checking the ARTHUR Web site for new games with the Dear Educator: World Neighborhood ® ® has been a proud sponsor of the Libby’s Juicy Juice ® theme. RTHUR since its debut in award-winning PBS series A ium 100% 1996. Like Arthur, Libby’s Juicy Juice, prem juice, is wholesome and loved by kids. itment to a RTHUR’s comm Libby’s Juicy Juice shares A world in which all children and cultures are appreciated. We applaud the efforts of PBS in producingArthur’s quality W orld educational television and hope that Neighborhood will be a valuable resource for teaching children to understand and reach out to one another. Enjoy! Libby’s Juicy Juice Contents About This Guide. 1 Around the Block . 2 Examine diversity within your community Around the World. 6 Everyday Life in Many Cultures: An overview of world diversity Delve Deeper: Explore a specific culture Dear Pen Pal . 10 Build personal connections through a pen pal exchange More Curriculum Connections . 14 Infuse your curriculum with global and cultural awareness Reflections . 15 Reflect on and share what you have learned Resources . 16 All characters and underlying materials (including artwork) copyright by Marc Brown.Arthur, D.W., and the other Marc Brown characters are trademarks of Marc Brown. About This Guide As children reach the early elementary years, their “neighborhood” expands beyond family and friends, and they become aware of a larger, more diverse “We live in a world in world. How are they similar and different from others? What do those which we need to share differences mean? Developmentally, this is an ideal time for teachers and providers to join children in exploring these questions. -

International Harvester Company
... - --- 11'1 .l.L.I 949 Complaint IN THE MATTER OF INTERNATIONAL HARVESTER COMPANY FINAL ORDER, OPINION , ETC. IN REGARD TO VIOLATION OF SEC. 5 OF THE FEDERAL TRADE COMMISSION ACT Docket 914Z Complaint, Oct. 10, 1980-Final Order Dec. , 1984 This Order affrms in part and reverses in part the 1982 Initial Decision ofthe Adminis- trative Law Judge C'AW") and orders that it be adopted as the "Findings of Fact and Conclusions of Law of the Commission, except as is inconsistent with the accompanying Opinion. " The ALl had ruled that a Chicago, Ill. manufacturer of farm machinery had violated Sec. 5 of the FTCA by failing to adequately disclose to consumers that its gasoline-powered tractors were subject to a safety hazard known as "fuel geysering," even though the company knew of the potential danger. While the Commission agreed that the company s failure to disclose the safety risk constituted an unfair trade practice, it ruled that, contrary to the AW's finding, the practice could not, as a matter of law , be considered deceptive since there was no representation , practice or omission likely to mislead consumers found in this case. Although the Commission ruled that the manufacturer has violated the FTCA, it upheld the ALJ's decision not to order further remedial action because the company no longer manufactures gasoline-powered tractors and because the company s 1980 voluntary notification program had already provided as much relief as could be expected from a Commission order. Appearances For the Commission: Richard H. Gateley, Michael Milgram, Rose- mary Rosso, Michael L.