Femur, Tibia and Fibula Bone Templates to Estimate Subject-Specific Knee
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Patellofemoral Syndrome: Evaluation & Management Scott Sevinsky MSPT
Patellofemoral Syndrome: Evaluation & Management Scott Sevinsky MSPT What is Patellofemoral Syndrome? Patellofemoral syndrome (PFS) is a term commonly used to describe a condition where the patella ‘tracks’ or glides improperly between the femoral condyles. This improper tracking causes pain in the anterior knee and may lead to degenerative changes or dislocation of the knee cap. To be more precise the term ‘anterior knee pain’ is suggested to encompass all pain-related problems of the anterior part of the knee. By excluding anterior knee pain due to intra-articular pathology, peripatellar tendinitis or bursitis, plica syndromes, Sinding Larsen’s disease, Osgood Schlatter’s disease, neuromas and other rarely occurring pathologies it is suggested that remaining patients with a clinical presentation of anterior knee pain could be diagnosed with PFPS. The term ‘patellofemoral’ is used as no distinction can be made as to which specific structure of the patella or femur is affected. The term ‘chondromalacia patellae’, defined at the beginning of the 20th century to describe pathological changes of the retropatellar cartilage,7,8 was for half a century, used as a synonym for the syndrome of patellofemoral pain. However, several studies during the last 2 decades have shown a poor correlation between articular cartilage damage and the still not well-defined pain mechanism of retropatellar pain. Review of Knee Anatomy 1. Femur – thigh bone; longest bone in the body. · Lateral femoral condyle larger than medial condyle & projects farther anteriorly. · Medial femoral condyle longer anterior to posterior · Distal surfaces are convex · Intercondylar (trochlear) notch: groove in which the patella glides or ‘tracks’ 2. -

Skier Tibia (Leg) Fractures
Skier Tibia (Leg) Fractures In years past, the prototypical ski fracture was sustained at the lower part of the outside of the leg in the region of the ankle. However, in the past 10 years, with the advent of the modern ski boots and improvements in binding, the most commonly seen lower leg skier fracture is the tibia (or shinbone) fracture. 10% of these fractures are associated with a collision. Thus, 90 % are associated with an isolated fall or noncontact type of injury, which is generally the result of binding malfunctions and inappropriate release. The most common mechanism leading to a tibia (leg) fracture is a forward fall. Risk factors for sustaining a skier tibia fracture include: beginners or novice skiers, less than 20 years of age, higher outdoor temperatures, and increased snow depth. Non-risk factors include ski lengths, icy conditions, and male versus female sex. The modern ski boot very closely resembles an extremely well padded short leg cast in the treatment of many orthopaedic lower extremity fractures. It of course goes to a much higher level than the former shorter boot top-level varieties. The binding release and designs have been based on the fracture strength of the adult tibia (shin) bone at the top of the modern ski boot. The treatment of most skier leg fractures includes a closed reduction and cast application for variable periods of time, with or without weight bearing allowed. However, severe misalignments of the bones can lead to later bony prominences that may be incompatible with snug, rigid, high fitting ski boots. -

Medial Lateral Malleolus
Acutrak 2® Headless Compression Screw System 4.7 mm and 5.5 mm Screws Supplemental Use Guide—Medial & Lateral Malleolus Acumed® is a global leader of innovative orthopaedic and medical solutions. We are dedicated to developing products, service methods, and approaches that improve patient care. Acumed® Acutrak 2® Headless Compression Screw System—4.7 mm and 5.5 mm This guide is intended for supplemental use only and is not intended to be used as a stand-alone surgical technique. Reference the Acumed Acutrak 2 Headless Compression Screw System Surgical Technique (SPF00-02) for more information. Definition Indicates critical information about a potential serious outcome to the Warning patient or the user. Indicates instructions that must be followed in order to ensure the proper Caution use of the device. Note Indicates information requiring special attention. Acumed® Acutrak 2® Headless Compression System—Supplemental Use Guide—Medial & Lateral Malleolus Table of Contents System Features ...........................................2 Surgical Techniques ........................................ 4 Fibula Fracture (Weber A and B Fractures) Surgical Technique: Acutrak 2®—5.5 .......................4 Medial Malleolus Surgical Technique: Acutrak 2®—4.7 ......................10 Ordering Information ......................................16 Acumed® Acutrak 2® Headless Compression System—Supplemental Use Guide—Medial & Lateral Malleolus System Features Headless screw design is intended to minimize soft tissue irritation D Acutrak 2 Screws Diameter -

Assessment, Management and Decision Making in the Treatment Of
Pediatric Ankle Fractures Anthony I. Riccio, MD Texas Scottish Rite Hospital for Children Update 07/2016 Pediatric Ankle Fractures The Ankle is the 2nd most Common Site of Physeal Injury in Children 10-25% of all Physeal Injuries Occur About the Ankle Pediatric Ankle Fractures Primary Concerns Are: • Anatomic Restoration of Articular Surface • Restoration of Symmetric Ankle Mortise • Preservation of Physeal Growth • Minimize Iatrogenic Physeal Injury • Avoid Fixation Across Physis in Younger Children Salter Harris Classification Prognosis and Treatment of Pediatric Ankle Fractures is Often Dictated by the Salter Harris Classification of Physeal Fractures Type I and II Fractures: Often Amenable to Closed Tx / Lower Risk of Physeal Arrest Type III and IV: More Likely to Require Operative Tx / Higher Risk of Physeal Arrest Herring JA, ed. Tachdjian’s Pediatric Orthopaedics, 5th Ed. 2014. Elsevier. Philadelphia, PA. ISOLATED DISTAL FIBULA FRACTURES Distal Fibula Fractures • The Physis is Weaker than the Lateral Ankle Ligaments – Children Often Fracture the Distal Fibula but…. – …ligamentous Injuries are Not Uncommon • Mechanism of Injury = Inversion of a Supinated Foot • SH I and II Fractures are Most Common – SH I Fractures: Average Age = 10 Years – SH II Fractures: Average Age = 12 Years Distal Fibula Fractures Lateral Ankle Tenderness SH I Distal Fibula Fracture vs. Lateral Ligamentous Injury (Sprain) Distal Fibula Fractures • Sankar et al (JPO 2008) – 37 Children – All with Open Physes, Lateral Ankle Tenderness + Normal Films – 18%: Periosteal -

Study Guide Medical Terminology by Thea Liza Batan About the Author
Study Guide Medical Terminology By Thea Liza Batan About the Author Thea Liza Batan earned a Master of Science in Nursing Administration in 2007 from Xavier University in Cincinnati, Ohio. She has worked as a staff nurse, nurse instructor, and level department head. She currently works as a simulation coordinator and a free- lance writer specializing in nursing and healthcare. All terms mentioned in this text that are known to be trademarks or service marks have been appropriately capitalized. Use of a term in this text shouldn’t be regarded as affecting the validity of any trademark or service mark. Copyright © 2017 by Penn Foster, Inc. All rights reserved. No part of the material protected by this copyright may be reproduced or utilized in any form or by any means, electronic or mechanical, including photocopying, recording, or by any information storage and retrieval system, without permission in writing from the copyright owner. Requests for permission to make copies of any part of the work should be mailed to Copyright Permissions, Penn Foster, 925 Oak Street, Scranton, Pennsylvania 18515. Printed in the United States of America CONTENTS INSTRUCTIONS 1 READING ASSIGNMENTS 3 LESSON 1: THE FUNDAMENTALS OF MEDICAL TERMINOLOGY 5 LESSON 2: DIAGNOSIS, INTERVENTION, AND HUMAN BODY TERMS 28 LESSON 3: MUSCULOSKELETAL, CIRCULATORY, AND RESPIRATORY SYSTEM TERMS 44 LESSON 4: DIGESTIVE, URINARY, AND REPRODUCTIVE SYSTEM TERMS 69 LESSON 5: INTEGUMENTARY, NERVOUS, AND ENDOCRINE S YSTEM TERMS 96 SELF-CHECK ANSWERS 134 © PENN FOSTER, INC. 2017 MEDICAL TERMINOLOGY PAGE III Contents INSTRUCTIONS INTRODUCTION Welcome to your course on medical terminology. You’re taking this course because you’re most likely interested in pursuing a health and science career, which entails proficiencyincommunicatingwithhealthcareprofessionalssuchasphysicians,nurses, or dentists. -

Free Vascularized Fibula Graft with Femoral Allograft Sleeve for Lumbar Spine Defects After Spondylectomy of Malignant Tumors Acasereport
1 COPYRIGHT Ó 2020 BY THE JOURNAL OF BONE AND JOINT SURGERY,INCORPORATED Free Vascularized Fibula Graft with Femoral Allograft Sleeve for Lumbar Spine Defects After Spondylectomy of Malignant Tumors ACaseReport Michiel E.R. Bongers, MD, John H. Shin, MD, Sunita D. Srivastava, MD, Christopher R. Morse, MD, Sang-Gil Lee, MD, and Joseph H. Schwab, MD, MS Investigation performed at Massachusetts General Hospital, Boston, Massachusetts Abstract Case: We present a 65-year-old man with an L4 conventional chordoma. Total en bloc spondylectomy (TES) of the involved vertebral bodies and surrounding soft tissues with reconstruction of the spine using a free vascularized fibula autograft (FVFG) is a proven technique, limiting complications and recurrence. However, graft fracture has occurred only in the lumbar spine in our institutional cases. We used a technique in our patient to ensure extra stability and support, with the addition of a femoral allograft sleeve encasing the FVFG. Conclusions: Our technique for the reconstruction of the lumbar spine after TES of primary malignant spinal disease using a femoral allograft sleeve encasing the FVFG is viable to consider. he treatment of primary malignant neoplasms of the spine mended a magnetic resonance imaging (MRI), but the request currently mainly relies on surgery, often in conjunction with was denied by the insurance company, and the patient T 1-3 radiotherapy .Totalen bloc spondylectomy (TES) is a widely underwent a course of physical therapy with no benefitand accepted surgical technique and has lower reported recurrence rates progression of back pain and radiculopathy. Four months compared with patients who undergo intralesional surgery3,4. -

Common Stress Fractures BRENT W
COVER ARTICLE PRACTICAL THERAPEUTICS Common Stress Fractures BRENT W. SANDERLIN, LCDR, MC, USNR, Naval Branch Medical Clinic, Fort Worth, Texas ROBERT F. RASPA, CAPT, MC, USN, Naval Hospital Jacksonville, Jacksonville, Florida Lower extremity stress fractures are common injuries most often associated with partic- ipation in sports involving running, jumping, or repetitive stress. The initial diagnosis can be made by identifying localized bone pain that increases with weight bearing or repet- itive use. Plain film radiographs are frequently unrevealing. Confirmation of a stress frac- ture is best made using triple phase nuclear medicine bone scan or magnetic resonance imaging. Prevention of stress fractures is most effectively accomplished by increasing the level of exercise slowly, adequately warming up and stretching before exercise, and using cushioned insoles and appropriate footwear. Treatment involves rest of the injured bone, followed by a gradual return to the sport once free of pain. Recent evidence sup- ports the use of air splinting to reduce pain and decrease the time until return to full par- ticipation or intensity of exercise. (Am Fam Physician 2003;68:1527-32. Copyright© 2003 American Academy of Family Physicians) tress fractures are among the involving repetitive use of the arms, such most common sports injuries as baseball or tennis. Stress fractures of and are frequently managed the ribs occur in sports such as rowing. by family physicians. A stress Upper extremity and rib stress fractures fracture should be suspected in are far less common than lower extremity Sany patient presenting with localized stress fractures.1 bone or periosteal pain, especially if he or she recently started an exercise program Etiology and Pathophysiology or increased the intensity of exercise. -

Luxating Patella
LUXATING PATELLA What is a luxating patella? The patella, or kneecap, is normally located in the center of the knee joint. The term luxating means, “out of place” or “dislocated”. Therefore, a luxating patella is a kneecap that moves out of its normal location. What causes this? The muscles of the thigh attach to the top of the kneecap. There is a ligament, the patellar ligament, running from the bottom of the kneecap to a point on the tibia just below the knee joint. When the thigh muscles contract, force is transmitted through the patella and patellar ligament to a point on the top of the tibia. This results in extension or straightening of the knee. The patella stays in the center of the leg because the point of attachment of the patellar ligament is on the midline and because the patella slides in a groove on the lower end of the femur (the bone between the knee and the hip). The patella luxates because the point of attachment of the patellar ligament is not on the midline of the tibia. It is almost always located too far medial (toward the middle of the body). As the thigh muscles contract, the force is pulled medial. After several months or years of this abnormal movement, the inner side of the groove in the femur wears down. Once the side of the groove wears down, the patella is then free to dislocate. When this occurs, the dog has difficulty bearing weight on the leg. It may learn how to kick the leg and snap the patella back into its normal location. -
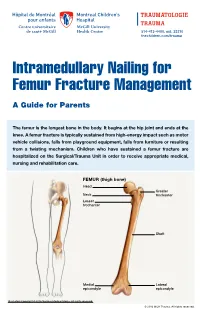
Intramedullary Nailing for Femur Fracture Management a Guide for Parents
514-412-4400, ext. 23310 thechildren.com/trauma Intramedullary Nailing for Femur Fracture Management A Guide for Parents The femur is the longest bone in the body. It begins at the hip joint and ends at the knee. A femur fracture is typically sustained from high-energy impact such as motor vehicle collisions, falls from playground equipment, falls from furniture or resulting from a twisting mechanism. Children who have sustained a femur fracture are hospitalized on the Surgical/Trauma Unit in order to receive appropriate medical, nursing and rehabilitation care. FEMUR (thigh bone) Head Greater Neck trochanter Lesser trochanter Shaft Medial Lateral epicondyle epicondyle Illustration Copyright © 2016 Nucleus Medical Media, All rights reserved. © 2016 MCH Trauma. All rights reserved. FEMUR FRACTURE MANAGEMENT The pediatric Orthopedic Surgeon will assess your child in order to determine the optimal treatment method. Treatment goals include: achieving proper bone realignment, rapid healing, and the return to normal daily activities. The treatment method chosen is primarily based on the child’s age but also taken into consideration are: fracture type, location and other injuries sustained if applicable. Prior to the surgery, your child may be placed in skin traction. This will ensure the bone is in an optimal healing position until it is surgically repaired. Occasionally, traction may be used for a longer period of time. The surgeon will determine if this management is needed based on the specific fracture type and/or location. ELASTIC/FLEXIBLE INTRAMEDULLARY NAILING This surgery is performed by the Orthopedic Surgeon in the Operating Room under general anesthesia. The surgeon will usually make two small incisions near the knee joint in order to insert two flexible titanium rods (intramedullary nails) Flexible through the femur. -
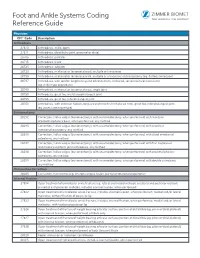
Foot and Ankle Systems Coding Reference Guide
Foot and Ankle Systems Coding Reference Guide Physician CPT® Code Description Arthrodesis 27870 Arthrodesis, ankle, open 27871 Arthrodesis, tibiofibular joint, proximal or distal 28705 Arthrodesis; pantalar 28715 Arthrodesis; triple 28725 Arthrodesis; subtalar 28730 Arthrodesis, midtarsal or tarsometatarsal, multiple or transverse 28735 Arthrodesis, midtarsal or tarsometatarsal, multiple or transverse; with osteotomy (eg, flatfoot correction) 28737 Arthrodesis, with tendon lengthening and advancement, midtarsal, tarsal navicular-cuneiform (eg, miller type procedure) 28740 Arthrodesis, midtarsal or tarsometatarsal, single joint 28750 Arthrodesis, great toe; metatarsophalangeal joint 28755 Arthrodesis, great toe; interphalangeal joint 28760 Arthrodesis, with extensor hallucis longus transfer to first metatarsal neck, great toe, interphalangeal joint (eg, jones type procedure) Bunionectomy 28292 Correction, hallux valgus (bunionectomy), with sesamoidectomy, when performed; with resection of proximal phalanx base, when performed, any method 28295 Correction, hallux valgus (bunionectomy), with sesamoidectomy, when performed; with proximal metatarsal osteotomy, any method 28296 Correction, hallux valgus (bunionectomy), with sesamoidectomy, when performed; with distal metatarsal osteotomy, any method 28297 Correction, hallux valgus (bunionectomy), with sesamoidectomy, when performed; with first metatarsal and medial cuneiform joint arthrodesis, any method 28298 Correction, hallux valgus (bunionectomy), with sesamoidectomy, when performed; with -

Unilateral Proximal Focal Femoral Deficiency, Fibular Aplasia, Tibial
The Egyptian Journal of Medical Human Genetics (2014) 15, 299–303 Ain Shams University The Egyptian Journal of Medical Human Genetics www.ejmhg.eg.net www.sciencedirect.com CASE REPORT Unilateral proximal focal femoral deficiency, fibular aplasia, tibial campomelia and oligosyndactyly in an Egyptian child – Probable FFU syndrome Rabah M. Shawky a,*, Heba Salah Abd Elkhalek a, Shaimaa Gad a, Shaimaa Abdelsattar Mohammad b a Pediatric Department, Genetics Unit, Ain Shams University, Egypt b Radio Diagnosis Department, Ain Shams University, Egypt Received 2 March 2014; accepted 18 March 2014 Available online 30 April 2014 KEYWORDS Abstract We report a fifteen month old Egyptian male child, the third in order of birth of healthy Short femur; non consanguineous parents, who has normal mentality, normal upper limbs and left lower limb. Limb anomaly; The right lower limb has short femur, and tibia with anterior bowing, and an overlying skin dimple. FFU syndrome; The right foot has also oligosyndactyly (three toes), and the foot is in vulgus position. There is lim- Proximal focal femoral ited abduction at the hip joint, full flexion and extension at the knee, limited dorsiflexion and plan- deficiency; tar flexion at the ankle joint. The X-ray of the lower limb and pelvis shows proximal focal femoral Fibular aplasia; deficiency, absent right fibula with shortening of the right tibia and anterior bowing of its distal Tibial campomelia; third. The acetabulum is shallow. He has a family history of congenital cyanotic heart disease. Oligosyndactyly Our patient represents most probably the first case of femur fibula ulna syndrome (FFU) in Egypt with unilateral right leg affection. -
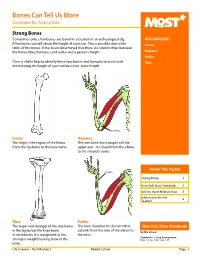
Bones Can Tell Us More Compiled By: Nancy Volk
Bones Can Tell Us More Compiled By: Nancy Volk Strong Bones Sometimes only a few bones are found in a location in an archeological dig. VOCABULARY A few bones can tell about the height of a person. This is possible due to the Femur ratios of the bones. It has been determined that there are relationships between the femur, tibia, humerus, and radius and a person’s height. Humerus Radius Here is a little help to identify these four bones and formulas to assist with Tibia determining the height of a person based on bone length. Humerus Femur: Humerus: The thigh is the region of the femur. The arm bone most people call the From the hip bone to the knee bone. upper arm. It is found from the elbow to the shoulder joints. Inside This Packet Radius Strong Bones 1 New York State Standards 1 Activity: Bone Relationships 2 Information for the Teacher 4 Tibia: Radius: The larger and stronger of the two bones The bone found in the forearm that New York State Standards in the leg below the knee bone. extends from the side of the elbow to Middle School In vertebrates It is recognized as the the wrist. Standard 4: Living Environment strongest weight bearing bone in the Idea 1: 1.2a, 1.2b, 1.2e, 1.2f body. Life Sciences - Post Module 3 Middle School Page 1 Activity: Bone Relationships MATERIALS NEEDED Skeleton Formulas: Tape Measure Bone relationship is represented by the following formulas: Directions and formulas P represents the person’s height. The last letter of each formula stands for the Calculator known length of the bone (femur, tibia, humerus, or radius) through measurement.