M Eeting Program
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Elixir, Urine and Hormone: a Socio-Cultural History of Qiushi (Autumn Mineral)*
EASTM 47 (2018): 19-54 Elixir, Urine and Hormone: A Socio-cultural History of Qiushi (Autumn Mineral)* Jing Zhu [ZHU Jing is an Associate Professor in the Department of Philosophy, East China Normal University. She received her Ph.D. in history of science at Peking University and in 2015-2016 was a visiting scholar at University of Pennsylvania. She has published three articles about qiushi and two articles about Chinese alchemy. Her paper “Arsenic Prepared by Chinese Alchemist-Pharmacists” was published in Science China Life Sciences. Her work spans historical research on Chinese alchemy, Chinese medicine and public understanding of science. In addition to presenting papers at national and international conferences, she has been invited to present her research among other places at the National Tsinghua University (Taiwan), Brown University, and the University of Pennsylvania. Contact: [email protected]] * * * Abstract: Traditional Chinese medicine has attracted the attention of pharmacologists because some of its remedies have proved useful against cancer and malaria. However, a variety of controversies have arisen regarding the difficulty of identifying and explaining the effectiveness of remedies by biomedical criteria. By exploring the socio-cultural history of qiushi (literally, ‘autumn mineral’), a drug prepared from urine and used frequently throughout Chinese history, I examine how alchemy, popular culture, politics and ritual influenced pre-modern views of the efficacy of the drug, and explore the sharp contrast between views of the drug’s * I especially wish to acknowledge the great help of Professor Nathan Sivin, who has read the complete manuscript and provided me with many critical comments. -

Mega Prize Winners VIKAS MAHAJAN SAMIR SHELAR UBAID AHMED K DILIP JAIN N KAVYA ASHISH AHIR RAJVARDHAN S LODHA FAIZAL KOTTIKOLLON
Mega Prize Winners VIKAS MAHAJAN SAMIR SHELAR UBAID AHMED K DILIP JAIN N KAVYA ASHISH AHIR RAJVARDHAN S LODHA FAIZAL KOTTIKOLLON SUMIT JAIN DHARMEN JADIA SAHIL TUTEJA SEJAL MODY Weekly Winners POONAMCHAND JAIN PRADEEP SHARMA SATISH WAGLE CYRUS P MISTRY JAGDISH PRASAD BANSAL SANDEEP JAIN SUSHIL KUMAR JAIN AAKRITI JAIN AYAAN SHETTY MITHLESH CHAUHAN SUNIL T KUKREJA SAMBHAJI KOLTE CHANDAN BHOWMICK SANJAY KAPUR NIKHIL MITTAL MOHIT RATHOD APURVA SHAH AMIT KOTHARI R BALAJI RANJITH S R DR GAURAV BASUTKAR SHARAD AGGARWAL CHETAN PRAJAPATI ANANT MEHTA MANIK AGGARWAL SUNIL NIKOSE MADAN DESHPANDE SUNIL SIPANI NITIN GUPTA AMIT RAMAN ARORA RAVI BHOSHAN SINGH AMIT HINDUJA ROHAN KOTHARI ATUL MARDIA BHOPAL RAJPUT ADITYA KUMAR RAI ACHAL KRISHNA SIDDHARTH MEHTA NITIN SINGHAL MUKUND PATEL SUBHAJYOT MUKHERJEE RAJ RANI RAJEEV SAMANT SEEMA ANIKET KUMAR BHARAT TAK SHASHI CHOUDHARY ALOK KHANNA RAJENDER SAHIL SHARMA PRASAD VOGOTI TRACEY LOBO ANUJ MEHTA G KHWAJA MUNSHI RUPAK HALDER SAUD MIRZA GOPINATH SUDARSHAN KUMAR V S SRINIVAS S G GOVIND ABHIJEET SINGH RAJEEV MARATHE SHUVAYU BISWAS VENUGOPAL SUNKU Mahendra Kumar Rao MONIKA KHUNGAR BIJESHWAREE SHAH SIREESHA GIRISH MANOHAR LAL KUMAWAT SANGITA SHARMA ROHIT GUPTA ANITESH GIRI GOSWAMI RAMAMOHAN W V ANJALI GUPTA KARAN KUMAR BHUTOR NITABEN PATEL KRISHNAKANT GUPTA HAFEEZHUSSAIN SYED IZATPAL SINGH YUSUF MOHAMMED JAVED SUMIT KAPOOR SOURAV SINGLA VIJAYA SAGAR REDDY RAHUL KAPOOR VARGHESE ISAAC AVINASH MISHRA R NAGESWARA RAO S DORAIRAJ NAVDEEP CHAWLA SUSHIL KUMAR JAIN SNEHA RANJAN CHOUDHUR SHARAD BANSAL KAMLESH MAHESWARI KAILASH -
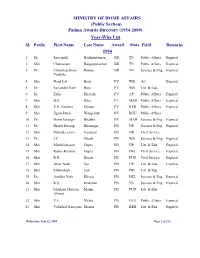
(Public Section) Padma Awards Directory (1954-2009) Year-Wise List Sl
MINISTRY OF HOME AFFAIRS (Public Section) Padma Awards Directory (1954-2009) Year-Wise List Sl. Prefix First Name Last Name Award State Field Remarks 1954 1 Dr. Sarvapalli Radhakrishnan BR TN Public Affairs Expired 2 Shri Chakravarti Rajagopalachari BR TN Public Affairs Expired 3 Dr. Chandrasekhara Raman BR TN Science & Eng. Expired Venkata 4 Shri Nand Lal Bose PV WB Art Expired 5 Dr. Satyendra Nath Bose PV WB Litt. & Edu. 6 Dr. Zakir Hussain PV AP Public Affairs Expired 7 Shri B.G. Kher PV MAH Public Affairs Expired 8 Shri V.K. Krishna Menon PV KER Public Affairs Expired 9 Shri Jigme Dorji Wangchuk PV BHU Public Affairs 10 Dr. Homi Jehangir Bhabha PB MAH Science & Eng. Expired 11 Dr. Shanti Swarup Bhatnagar PB UP Science & Eng. Expired 12 Shri Mahadeva Iyer Ganapati PB OR Civil Service 13 Dr. J.C. Ghosh PB WB Science & Eng. Expired 14 Shri Maithilisharan Gupta PB UP Litt. & Edu. Expired 15 Shri Radha Krishan Gupta PB DEL Civil Service Expired 16 Shri R.R. Handa PB PUN Civil Service Expired 17 Shri Amar Nath Jha PB UP Litt. & Edu. Expired 18 Shri Malihabadi Josh PB DEL Litt. & Edu. 19 Dr. Ajudhia Nath Khosla PB DEL Science & Eng. Expired 20 Shri K.S. Krishnan PB TN Science & Eng. Expired 21 Shri Moulana Hussain Madni PB PUN Litt. & Edu. Ahmed 22 Shri V.L. Mehta PB GUJ Public Affairs Expired 23 Shri Vallathol Narayana Menon PB KER Litt. & Edu. Expired Wednesday, July 22, 2009 Page 1 of 133 Sl. Prefix First Name Last Name Award State Field Remarks 24 Dr. -

St James Infirmary Guide.Pdf
The St. James Infirmary is an Occupational Safety & Health Clinic for Sex Workers founded by COYOTE--Call Off Your Old Tired Ethics, and is a joint project between Exotic Dancers Alliance and the STD (Sexually Transmitted Disease) Prevention and Control Services of the City and County of San Francisco Department of Public Health. Our mission is to provide non-judgmental and compassionate health care and social services for all Sex Workers while preventing occupational illnesses and injuries throughout the sex industry. We provide services for current, former, and transitioning Street and Survival Sex Workers, Escorts, Sensual Massage workers, Erotic Performers and Entertainers, Exotic Dancers, Peep Show workers, Bondage/ Dominatrix/Sado-Masochism (BDSM) workers, Adult Film actors, Nude Models, Internet Pornography workers, Phone Sex operators, and Sex Toy Store workers. HOW TO USE THIS GUIDE This resource guide was prepared by Sex Workers for Sex Workers, and is produced with funds received from The California Endowment, the Franklin Benevolent Corporation and the SFDPH AIDS office. The information throughout the following pages is meant to provide you with helpful tips so that you can make informed choices about your health and well-being. Organizations with a © have received In-Service trainings from St. James Infirmary staff and/or collaborated and/or networked with us to provide comprehensive services to Sex Workers. We welcome your comments and suggestions about any of the following information, and we hope to see you soon at the -

Prospectus Symp19-8-2019.Indd
EXHIBITOR & PROMOTIONAL PARTNERSHIP PROSPECTUS TH INTERNATIONAL PROSTATE CANCER AND UROLOGIC ONCOLOGY SYMPOSIUM NOVEMBER 7-9, 2019 NEW YORK, NY MOUNTSINAIUROLOGYCME.ORG HIGHLIGHTS • Over 100 world-renowned faculty • Engaging point-counterpoint debates and • Live 3-D surgical demonstrations, including panel discussion prostate, kidney, bladder procedures • Global exposure with international web streaming of select sessions and events Welcome to the Fourth International Prostate Cancer and Urologic Oncology Symposium. EDUCATIONAL HIGHLIGHTS • Live 3-D surgery transmission covering complex urologic surgery • Comprehensive didactic lectures from the world’s leading experts in urologic oncology • Robust review of the latest advances in surgical techniques and management of prostate, kidney, and bladder cancer • Personal interaction with world-class faculty • Stimulating point-counterpoint debates on timely topics DAILY HIGHLIGHTS PROSTATE DAY 1 THUR. 11/7/19 PROSTATE DAY 2 FRI. 11/8/19 KIDNEY & BLADDER SAT. 11/9/19 • Advanced imaging in genomics • Innovations in the management of • New progress in pre-operative and for prostate cancer castrate-sensitive prostate cancer intra-operative imaging, 3-D • Biomarkers and immunotherapy modelling, and ultra sound • Focus on radiomics and guidance for renal tumors radiogenomics • Update on clinical trials for castrate-resistant treatments for • Novel therapeutics in the • Utility of imaging and artificial prostate cancer management of metastatic renal intelligence in prostate cancer cell carcinomas: -

Envases Y Embalajes En Tunez
Oficina Económica y Comercial de la Embajada de España en Túnez El mercado de envases y embalajes en Túnez Notas Sectoriales El mercado de envases y embalajes en Túnez Esta nota ha sido elaborada por Inés Rentería bajo la supervisión de la Oficina Económica y Comercial de la Embajada de España en Túnez Septiembre 2008 Notas Sectoriales EL MERCADO DE ENVASES Y EMBALAJES EN TÚNEZ ÍNDICE CONCLUSIONES 4 I. DEFINICION DEL SECTOR 5 1. Delimitación del sector 5 II. INTERCAMBIOS COMERCIALES 8 III. ANÁLISIS CUALITATIVO 11 1. Producción 11 2. Obstáculos comerciales 13 3. Requisitos medioambientales del producto 21 4. Medios de pago y contratos comerciales 22 IV. ANÁLISIS DEL COMERCIO 28 1. Análisis cuantitativo 28 2. Análisis cualitativo 32 V. ANÁLISIS DE LA DEMANDA 35 1. Evaluación del volumen de la demanda 35 2. Estructura del mercado 38 3. Factores asociados a la decisión de compra 39 4. Percepción del producto español 39 VI. ANEXOS 41 1. Empresas 41 2. Ferias 76 Oficina Económica y Comercial de la Embajada de España en Túnez 3 EL MERCADO DE ENVASES Y EMBALAJES EN TÚNEZ CONCLUSIONES En cualquier economía, el sector del envase y embalaje es un sector indicador de la actividad económica general. En el caso de Túnez, se está desarrollando rápidamente, como su eco- nomía. En el sector, sin embargo, no todas las ramas van a la misma velocidad. Los subsec- tores de mayor crecimiento en los últimos años han sido los de embalaje de madera y metal. Papel y cartón, plástico y vidrio siguen en crecimiento sostenido y moderado. -

Top Ten 2006-U
SportOlimpico rev. 28-9-2012 TOP TEN 2006 – UOMINI 100 METRI 10”27 -0,9 Andrew Howe 12-5-85 1) Isili 2 Set 10”28 –04 10”30 1,5 Simone Collio 27-12-79 7) Gateshead 11 Giu 10”20 –04 10”33 1,0 Koura Fantoni Kaba 28-8-84 3) Valencia 27 Mag 10”28 –04 10”33 1,0 Rosario La Mastra 2-1-84 1) Rieti 21 Lug 10”66 –05 10”35 1,9 Massimiliano Donati 16-6-79 b1) Roma 17 Giu 10”37 –03 10”35 1,1 Marco Torrieri 14-5-78 1) Roma 17 Giu 10”22 –02 10”36 1,1 Luca Verdecchia 24-5-78 1) Fabriano 17 Giu 10”33 –03 10”37 1,3 Stefano Anceschi 18-6-84 1) Bellinzona 26 Ago 10”40 –05 10”39 0,0 Francesco Scuderi 4-10-77 F-4) Ginevra 11 Giu 10”19 –00 10”42 1,3 Maurizio Checcucci 26-2-74 1) Recanati 1 Lug 10”27 –01 -[10] Vento favorevole oltre 2 m/sec.: 10”34w 3.8 Stefanio Tobia 23-6-86 1) Donnas 18 Giu Juniores 10”49 1,6 Carlo Fadda (19) 21-1-87 1) Sassari 17 Giu 10”54 0,5 Matteo Galvan (18) 24-8-88 1) Rieti 21 Lug 10”58 1,6 Gavino Giacomo Dettori (19) 20-4-87 2) Sassari 17 Giu 10”82 –05 Vento favorevole oltre 2 m/sec.: 10”56w 2,6 Francesco Garzia (19) -87 1) Camerino 20 Giu 10”62w 3,3 Marco Lo Muscio (19) 2-10-87 1) Donnas 18 Giu 10”64w 3,1 Luca Berti Rigo (18) 4-6-88 3) Tunisi-Radés 5 Ago Appendice: 60 Metri – Indoor 6”66 Stefano Dacastello 17-2-80 1) Bra 28 Gen 6”66 Koura Fantoni-Kaba 28-8-84 b1) Aosta 4 Feb 200 METRI 20”66 0,1 Francesco Scuderi 4-10-77 1) Palermo 18 Giu 20”95 –02 20”70 0,2 Koura Fantoni Kaba 28-8-84 2) Doha 12 Mag 20”58 –05 20”82 -0,5 Alessandro Cavallaro 22-2-80 b3) Gôteborg 9 Ago 20”42 –02 20”86 0,3 Stefano Anceschi 18-6-84 4) Torino 6 Giu 21”22 –05 20”87-Jr 0,7 Matteo Galvan (18) 24-8-88 1) Rieti 23 Lug 21”14 –05 20”92 0,2 Marco Torrieri 14-5-78 2) Avellino 14 Giu 20”38 –01 21”01 0,1 Marco Cuneo 2-7-80 1) Sesto Fiorentino 15 Giu 20”84 –03 21”09 0,2 Massimiliano Donati 16-6-79 1) Rieti 6 Set 20”80 –99 21”17 1,0 Mario A. -

An International Journal for Students of Theological and Religious Studies Volume 36 Issue 2 July 2011
An International Journal for Students of Theological and Religious Studies Volume 36 Issue 2 July 2011 EDITORIAL: Generational Conflict in Ministry 180 D. A. Carson MINORITY REPORT: A Word to the Conscience 183 Carl Trueman Is the Reformation Over? John Calvin, Roman Catholicism, 185 and Contemporary Ecumenical Conversations Scott M. Manetsch Intrinsic Canonicity and the Inadequacy of the 203 Community Approach to Canon-Determination John C. Peckham Canon as Tradition: The New Covenant and the 216 Hermeneutical Question Mark R. Saucy Not Ashamed! The Sufficiency of Scripture for 238 Public Theology Dan Strange A Preacher’s Decalogue 261 Sinclair B. Ferguson Book Reviews 269 DESCRIPTION Themelios is an international evangelical theological journal that expounds and defends the historic Christian faith. Its primary audience is theological students and pastors, though scholars read it as well. It was formerly a print journal operated by RTSF/UCCF in the UK, and it became a digital journal operated by The Gospel Coalition in 2008. The new editorial team seeks to preserve representation, in both essayists and reviewers, from both sides of the Atlantic. Themelios is published three times a year exclusively online at www.theGospelCoalition.org. It is presented in two formats: PDF (for citing pagination) and HTML (for greater accessibility, usability, and infiltration in search engines). Themelios is copyrighted by The Gospel Coalition. Readers are free to use it and circulate it in digital form without further permission (any print use requires further written permission), but they must acknowledge the source and, of course, not change the content. EDITORS BOOK ReVIEW EDITORS Systematic Theology and Bioethics Hans Madueme General Editor: D. -

In the News September 30, 2016
From: ITNDaily on behalf of Werle, Laura Subject: Mount Sinai In the News - September 30, 2016 Date: Friday, September 30, 2016 2:06:09 PM Attachments: ATT00002.txt In the News September 30, 2016 Nature – September 30 Inflammation in Patients With Cushing Disease — Claire Greenhill Circulating levels of proinflammatory cytokines are increased in patients with Cushing disease, both during active disease and after remission, according to new data published in Clinical Endocrinology. These findings demonstrate that cytokine levels are increased in patients after remission of Cushing disease, which could explain the persistent increased risk of cardiovascular-related death. The study, led by Eliza Geer, MD, a professor of endocrinology at the Icahn School of Medicine at Mount Sinai, notes that more studies are needed to determine why levels of IL-6 and IL-1β remain elevated after remission of Cushing disease. - Eliza Geer, MD, Associate Professor, Medicine, Endocrinology, Diabetes and Bone Disease, Neurosurgery, Icahn School of Medicine at Mount Sinai Learn more: http://www.nature.com/nrendo/journal/vaop/ncurrent/full/nrendo.2016.170.html WCBS – September 29 ‘Artificial Pancreas’ Expected To Make Life Easier, Healthier For Type 1 Diabetes Patients — Max Gomez A groundbreaking new device could change the lives of millions of people living with diabetes. Federal regulators just approved an artificial pancreas that can monitor and administer insulin. The key is a computer algorithm that does those calculations faster and more accurately than a patient can. “It looks at the numbers from a moment to moment basis, and it will proactively predict what is going to happen next and make clinical decisions that cannot be done at that rate. -

Release List of up to 30 Players
Release list of up to 30 players Each association’s release list of up to 30 players was received by 11 May 2010 as per article 26 of the Regulations for the 2010 FIFA World Cup South Africa™. Mandatory rest period for players on the release list is from 17-23 May 2010 (except players involved in the UEFA Champions League final on 22 May). Each association must send FIFA a final list of no more than 23 players by 24.00 CET on 1 June 2010. Final list is limited to players on the release list submitted on 11 May 2010. Final list of 23 players will be published on FIFA.com at 12.00 CET on 4 June. Injured players may be replaced up to 24 hours before a team’s first match. Replacement players are not limited to the release list of up to 30 players. Liste de joueurs à libérer Les listes de joueurs à libérer (comportant jusqu’à 30 joueurs) ont été reçues de chaque association membre participante avant le 11 mai 2010 conformément à l’art. 26 du Règlement de la Coupe du Monde de la FIFA, Afrique du Sud 2010. La période de repos obligatoire à observer par les joueurs figurant sur la liste de joueurs à libérer (à l’exception des joueurs participant à la finale de la Ligue des Champions de l’UEFA le 22 mai) est du 17 au 23 mai 2010. Chaque association doit envoyer à la FIFA une liste définitive comportant un maximum de 23 joueurs au plus tard le 1er juin 2010 à minuit (heure centrale européenne). -

Final Program
14th ACS/IEEE International Conference on Computer Systems and Applications AICCSA 2017 October 30th to November 3rd, 2017 Final Program 1 14th ACS/IEEE International Conference on Computer Systems and Applications AICCSA 2017 October 30th to November 3rd, 2017 Final Program 2 14th ACS/IEEE International Conference on Computer Systems and Applications AICCSA 2017 October 30th to November 3rd, 2017 Final Program Monday 30-Oct -2017 12:30 - Onsite registration 17:00 Workshop Online Social Networks Technologies Chair: Dr. Muder Almi'ani Workshop Manal Abdo Farhan Saif, Ahmed Tlili, Fathi Essalmi and Mohamed Jemni. Facebook as a OSNT learning tool in classrooms: A case study (Sultan) Amina Amara, Mohamed Ali Hadj Taieb and Mohamed Ben Aouicha. Identifying i-bridge across online social networks Samiha El Hamali, Latifa Mahdaoui and Srikanth Podatharapu. Using Social Networks to Improve Processes in Business Education: A Study Case Workshop Arabic Natural Language Processing Chair: Prof. Ashraf Elnagar Ahmed Omer and Michael Oakes. Arud, the Metrical System of Arabic Poetry, as a Feature Set for Authorship Attribution Workshop Houcemeddine Turki, Denny Vrandečić, Helmi Hamdi and Imed Adel. Using WikiData to ANLP create a multi-lingual multi-dialectal dictionary for Arabic dialects (OIZIR) Ashraf Elnagar, Omar Einea and Leena Lulu. Comparative Study of Sentiment Classification for Automated Translated Latin Reviews into Arabic 13:30- Diab Abuaiadah, Dileep Rajendran and Mustafa Jarrar. Clustering Arabic Tweets for 16:00 Sentiment Analysis Workshop Big Data and Social Networking Management and Security Chair: Dr. Mohammad Alsmirat Workshop Olfa Mannai, Rabie Becheikh, Rhouma Rhouma and Safya Belghith. A Novel Family of BSDN Strong S-box Based on Ikeda Map and T-function (ETTEJ) Asma Chader, Hamid Haddadou and Walid Khaled Hidouci. -

Page 1 Soccer Players from Japan
Soccer Players from Japan - Free Printable Wordsearch MAKOTOHASEBE A A RYANGYONGGI K DTAKUYAYAMADAYUT ONAGATOMOI E CHONGYONGDE AR MKAZUOHONMA WA IAKIRANARAHASHI AK RNAOHIROISHIKAWA GA SARYASUH ITOENDOA JJ ATY KAZUHIROMURAKAMIA IU NSTO EBADIOI UKKN TUOI ASUMNJTH SDIEI OTSSYC ETKADONOSTA NNC SOHUH ATASYHIMIA IOGH UMIUII TSAAUEHADANM SROI YCIHNCMG URKKSMTOA UINI AHCIHSHNAF IHUAAHARKHK NAN SIHRIEUOIEEH SAKSKIAA EIKA UDIOTDIJKKKADI EJAHTYV SSAM YAHHAEIAETOEAG NIIIPAEA HMO UIISAKOCTONKMI IRKMTUANNKIUT KSRETAHHSAHAH OIYEASRAA ZRO IUOITSAI SKSNAOHKAKG TAAK KKYGOHSRIO ADOYSAKOUMKIAWRA OEAORIHONT MOUHNISYIASA Z NMSNIHIMUE NUEKIYSOANA U NAUAIMAKD OARITOHGT GWN OTDRROKI IJAOUO AAJAO SAAATIHJ NBONY IKOR UZNON AEKGA AI IAOI HUHN GI KHI BAO I ISR OK O KUNISHIGE KAMAMOTO HIDEO HASHIMOTO TAKUYA TAKAGI CHONG YONG DE ATSUSHI YANAGISAWA NORIHIRO NISHI TAKASHI USAMI MAKOTO HASEBE KAZUHIRO MURAKAMI SEIICHIRO MAKI SOTA HIRAYAMA TAKUYA YAMADA TOSHIHIRO HATTORI HAJIME HOSOGAI KEISUKE HONDA PARK KANG JO AKINORI NISHIZAWA YASUYUKI KONNO RYANG YONG GI AN YOUNG HAK SHUNSUKE NAKAMURA KENGO NAKAMURA ADEMIR SANTOS KAZUNORI IIO MICHIHIRO YASUDA DAISUKE SAKATA YASUHITO ENDO JONG TAE SE NAOHIRO ISHIKAWA TAKASHI HIRANO TAKEFUSA KUBO KAZUO HONMA HIDETOSHI NAKATA DAISUKE MATSUI YUICHI KOMANO SHINJI ONO AKIRA NARAHASHI SEIGO NARAZAKI DIDO HAVENAAR AKIRA KAJI JUNICHI INAMOTO YUTO NAGATOMO ATSUTO UCHIDA RI HAN JAE DAIKI TAKAMATSU YOSHITO OKUBO SHINJI KAGAWA KISHO YANO RYOICHI MAEDA YUKI ABE Free Printable Wordsearch from LogicLovely.com. Use freely for any use, please give a