Eortc-1307-Bcg) (Big5-13) (Tesaro Pr-30-5010-C)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

List of Medicinal Products Under Additional Monitoring
26 October 2018 EMA/245297/2013 Rev.60 Inspections & Human Medicines Pharmacovigilance List of medicinal products under additional monitoring Related Information: Additional monitoring explained: http://www.ema.europa.eu/ema/index.jsp?curl=pages/special_topics/document_listing/document_listing_000365.jsp Good Pharmacovigilance Practice Module on additional monitoring: http://www.ema.europa.eu/ema/index.jsp?curl=pages/regulation/document_listing/document_listing_000345.jsp To note: All products added to the list in September 2018 are shown in red font. All products removed from the list are shown with a strikethrough for the period of one month after which they are excluded. Date of Product name Active Substance (s) Reason (s) on list Marketing Authorisation Holder (s) Link to Product Information Inclusion http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/ Abasaglar (previously Abasria) Insulin glargine New biological Eli Lilly Nederland B.V. 002835/human_med_001790.jsp&mid=WC0b01ac058001d124 October 2014 Acarizax (also known in some EU countries as MITIZAX) Standardised allergen extract from house dust mites New Biological ALK-Abelló A/S https://portal.dimdi.de/amispb/doc/pei/Web/2613318-spcde-20170401.pdf May 2016 http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/ Accofil Filgrastim New biological Accord Healthcare Limited 003956/human_med_001798.jsp&mid=WC0b01ac058001d124 October 2014 http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/ Adcetris Brentuximab vedotin -
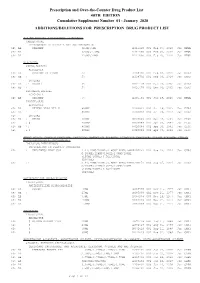
January 2020: Additions and Deletions to the Drug Product List
Prescription and Over-the-Counter Drug Product List 40TH EDITION Cumulative Supplement Number 01 : January 2020 ADDITIONS/DELETIONS FOR PRESCRIPTION DRUG PRODUCT LIST ACETAMINOPHEN; HYDROCODONE BITARTRATE TABLET;ORAL HYDROCODONE BITARTRATE AND ACETAMINOPHEN >A> AA XIROMED 325MG;5MG A 211690 001 Feb 07, 2020 Jan NEWA >A> AA 325MG;7.5MG A 211690 002 Feb 07, 2020 Jan NEWA >A> AA 325MG;10MG A 211690 003 Feb 07, 2020 Jan NEWA ACYCLOVIR CREAM;TOPICAL ACYCLOVIR >D> AB PERRIGO UK FINCO 5% A 208702 001 Feb 04, 2019 Jan CHRS >A> AB ! 5% A 208702 001 Feb 04, 2019 Jan CHRS ZOVIRAX >D> AB +! BAUSCH 5% N 021478 001 Dec 30, 2002 Jan CHRS >A> AB + 5% N 021478 001 Dec 30, 2002 Jan CHRS OINTMENT;TOPICAL ACYCLOVIR >A> AB XIROMED 5% A 201501 001 Jan 29, 2020 Jan NEWA TABLET;ORAL ACYCLOVIR >D> AB HETERO LABS LTD V 800MG A 203834 002 Oct 29, 2013 Jan CHRS >A> AB ! 800MG A 203834 002 Oct 29, 2013 Jan CHRS >D> ZOVIRAX >D> AB + MYLAN 400MG N 020089 001 Apr 30, 1991 Jan DISC >A> + @ 400MG N 020089 001 Apr 30, 1991 Jan DISC >D> AB +! 800MG N 020089 002 Apr 30, 1991 Jan DISC >A> + @ 800MG N 020089 002 Apr 30, 1991 Jan DISC AMINO ACIDS; CALCIUM CHLORIDE; DEXTROSE; MAGNESIUM SULFATE; POTASSIUM CHLORIDE; SODIUM ACETATE; SODIUM GLYCEROPHOSPHATE; SOYBEAN OIL EMULSION;INTRAVENOUS PERIKABIVEN IN PLASTIC CONTAINER >D> + FRESENIUS KABI USA 2.4%;20MG/100ML;6.8GM/100ML;68M N 200656 003 Aug 25, 2014 Jan CHRS G/100ML;124MG/100ML;170MG/100ML ;105MG/100ML;3.5GM/100ML (2400ML) >A> +! 2.4%;20MG/100ML;6.8GM/100ML;68M N 200656 003 Aug 25, 2014 Jan CHRS G/100ML;124MG/100ML;170MG/100ML -

Final Belgian List
Final Belgian list: (Last update 07/02/2019) Marketing name Active substance MAH Reason on list ABASAGLAR (ABASRIA previously)INSULINE GLARGINE ELI LILLY REGIONAL OPERATIONS GMBH New Biological ACARIZAX STANDARDIZED ALLERGEN EXTRACT FROM HOUSE DUSTALK-ABELLO MITES A/S New Biological ACCOFIL FILGASTRIM ACCORD HEALTHCARE LIMITED New Biological ADCETRIS BRENTUXIMAB VEDOTINE TAKEDA GLOBAL R&D CENTRE (EUROPE) LTD New AS, Cond Auth, PASS ADEMPAS RIOCIGUAT BAYER PHARMA New AS ADYNOVI RURIOCTOCOG ALFA PEGOL BAXALTA INNOVATIONS GMBH New AS, PASS AIMOVIG ERENUMAB NOVARTIS EUROPHARM LIMITED New AS, New Biological AFSTYLA LONOCTOCOG ALFA CSL BEHRING GmbH New AS STANDARDIZED ALLERGEN EXTRACT FROM HOUSE AITARO ALK-ABELLO A/S New Biological DUST MITES AKYNZEO NETUPITANT, PALONOSETRON HELSINN BIREX PHARMACEUTICALS LTD New AS ALECENSA ALECTINIB ROCHE REGISTRATION LIMITED New AS, Cond Auth ALOFISEL DAVASTROCEL TiGenix SAU New AS ALPHA-RIX TETRA INFLUENZA VACCINE INACTIVATED GLAXOSMITHKLINE BIOLOGICALS S.A. New Biological ALPIVAB PERAMIVIR BIOCRYST UK LTD New AS ALPROLIX EFTRENOCOG ALFA BIOGEN IDEC LTD New AS ALUNBRIG BRIGATINIB TAKEDA PHARMA A/S New AS AMGEVITA ADALIMUMAB AMGEN EUROPE BV New biological STANDARDIZED ALLERGEN EXTRACT FROM HOUSE AMITEND ALK-ABELLO A/S New Biological DUST MITES AMITIZA LUBIPROSTON Sucampo Pharma Europe Ltd New AS ANORO GLAXO GROUP LTD New AS, PASS UMECLIDINIUM BROMIDE, VILANTEROL TRIFENATATE APLEEK ETHINYL ESTRADIOL/GESTODENE BAYER PHARMA AG PASS ATRIANCE NELARABINE 5.00 MG/ML GLAXO GROUP LTD Auth under excep circumstances -

Antibodies to Watch in 2021 Hélène Kaplona and Janice M
MABS 2021, VOL. 13, NO. 1, e1860476 (34 pages) https://doi.org/10.1080/19420862.2020.1860476 PERSPECTIVE Antibodies to watch in 2021 Hélène Kaplona and Janice M. Reichert b aInstitut De Recherches Internationales Servier, Translational Medicine Department, Suresnes, France; bThe Antibody Society, Inc., Framingham, MA, USA ABSTRACT ARTICLE HISTORY In this 12th annual installment of the Antibodies to Watch article series, we discuss key events in antibody Received 1 December 2020 therapeutics development that occurred in 2020 and forecast events that might occur in 2021. The Accepted 1 December 2020 coronavirus disease 2019 (COVID-19) pandemic posed an array of challenges and opportunities to the KEYWORDS healthcare system in 2020, and it will continue to do so in 2021. Remarkably, by late November 2020, two Antibody therapeutics; anti-SARS-CoV antibody products, bamlanivimab and the casirivimab and imdevimab cocktail, were cancer; COVID-19; Food and authorized for emergency use by the US Food and Drug Administration (FDA) and the repurposed Drug Administration; antibodies levilimab and itolizumab had been registered for emergency use as treatments for COVID-19 European Medicines Agency; in Russia and India, respectively. Despite the pandemic, 10 antibody therapeutics had been granted the immune-mediated disorders; first approval in the US or EU in 2020, as of November, and 2 more (tanezumab and margetuximab) may Sars-CoV-2 be granted approvals in December 2020.* In addition, prolgolimab and olokizumab had been granted first approvals in Russia and cetuximab saratolacan sodium was first approved in Japan. The number of approvals in 2021 may set a record, as marketing applications for 16 investigational antibody therapeutics are already undergoing regulatory review by either the FDA or the European Medicines Agency. -

Available Medications and Supplies
Available Medications and Supplies * Denotes "Restricted Name Strength Units Dosage Form Generic Name Program Name AWP ARP Medications 8-MOP 10 MG CAPSULE(S) METHOXSALEN Valeant Patient Assistance Program $1,843.80 $2,120.37 ABACAVIR (BRAND: ZIAGEN) 300 MG TABLET(S) ABACAVIR Xubex Preferred Network Program $603.33 $693.83 ABACAVIR SULFATE, LAMIVUDINE AND ZIDOVUDINE (BRAND: TRIZIVIR) 300-150-300 MG-MG-MG TABLET(S) ABACAVIR SULFATE, Xubex Preferred Network Program $1,738.46 $1,999.23 LAMIVUDINE AND ZIDOVUDINE ABELCET 5 MG/ML (20 ML) MG AMPHOTERICIN B LIPID Sigma-Tau Patient Assistance Program $240.00 $276.00 COMPLEX ABILIFY * 1 MG/ML (150 ML) ML ARIPIPRAZOLE Bristol-Myers Squibb Patient Assistance $1,177.87 $1,354.55 Foundation, Inc. * 2 MG TABLET(S) ARIPIPRAZOLE Bristol-Myers Squibb Patient Assistance $1,070.36 $1,230.91 Foundation, Inc. * 20 MG TABLET(S) ARIPIPRAZOLE Bristol-Myers Squibb Patient Assistance $5,045.42 $5,802.23 Foundation, Inc. * 5 MG TABLET(S) ARIPIPRAZOLE Bristol-Myers Squibb Patient Assistance $3,567.66 $4,102.81 Foundation, Inc. ABILIFY * 10 MG TABLET(S) ARIPIPRAZOLE Bristol-Myers Squibb Patient Assistance $3,567.66 $4,102.81 Foundation, Inc. * 15 MG TABLET(S) ARIPIPRAZOLE Bristol-Myers Squibb Patient Assistance $3,567.66 $4,102.81 Foundation, Inc. Report Run: 04/19/16 10:53 AM Page 1 of 377 Available Medications and Supplies * Denotes "Restricted Name Strength Units Dosage Form Generic Name Program Name AWP ARP Medications ABILIFY * 30 MG TABLET(S) ARIPIPRAZOLE Bristol-Myers Squibb Patient Assistance $5,045.42 $5,802.23 Foundation, Inc. -

GSK Delivers Q2 Sales of £7.6 Billion -2% AER, -3% CER (Pro-Forma -10% CER*) Total EPS 45.5P >100% AER; >100% CER; Adjusted EPS 19.2P -37% AER, -38% CER
UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 Form 6-K REPORT OF FOREIGN PRIVATE ISSUER PURSUANT TO RULE 13a-16 OR 15d-16 UNDER THE SECURITIES EXCHANGE ACT OF 1934 For the month of July 2020 Commission File Number 001-15170 GlaxoSmithKline plc (Translation of registrant's name into English) 980 Great West Road, Brentford, Middlesex, TW8 9GS (Address of principal executive office) Indicate by check mark whether the registrant files or will file annual reports under cover of Form 20-F or Form 40-F. Form 20-F . .X. Form 40-F . Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(1): ____ Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(7): ____ Issued: Wednesday, 29 July 2020, London U.K. GSK delivers Q2 sales of £7.6 billion -2% AER, -3% CER (Pro-forma -10% CER*) Total EPS 45.5p >100% AER; >100% CER; Adjusted EPS 19.2p -37% AER, -38% CER Financial and product highlights ● Reported Group sales £7.6 billion -2% AER, -3% CER (Pro-forma -10% CER*; -8% CER excluding divestments/brands under review). Pharmaceuticals £4.1 billion -5% AER, -5% CER; Vaccines £1.1 billion -29% AER, -29% CER; Consumer Healthcare £2.4 billion +25% AER, +25% CER (Pro-forma -6% CER) ● H1 Reported group sales £16.7 billion 8% AER, 8% CER (Pro-forma flat CER*; +1% CER excluding divestments/brands under review) ● Sales decline in Q2 2020 reflects expected disruption from COVID-19, particularly in Vaccines as well as destocking from Q1 2020 in Pharmaceuticals and Consumer Healthcare ● Total Respiratory sales £883 million +17% AER, +16% CER. -

October Through December 2013
October through December 2013 precautions about the risk of blood clots and severe Updated Warnings—Current Drugs narrowing of blood vessels, revise recommendations about dosage and administration of Iclusig, and update the patient Medication Guide. We are also requiring a risk Mass Destruction Muscle Growth—Safety Risk: The evaluation and mitigation strategy (REMS). In addition, FDA is advising consumers to immediately stop using a the manufacturer of Iclusig, ARIAD Pharmaceuticals, must product called Mass Destruction, marketed as a dietary conduct postmarket investigations to further characterize supplement for muscle growth. The product is labeled to the drug’s safety and dosing. contain at least one synthetic anabolic steroid and has been On October 31, 2013, FDA requested and ARIAD agreed to linked to at least one reported serious illness. The product’s voluntarily suspend marketing of Iclusig. FDA’s request ingredients are undergoing further analysis by the FDA. resulted from FDA’s investigation, which revealed a steady Liver injury is generally known to be a possible outcome of increase in the number of serious vascular occlusion events using products that contain anabolic steroids and steroid- identified through continued safety monitoring of the drug. like substances. In general, anabolic steroids may cause This observation represented a significant change in the other serious long-term consequences in women, men, and safety profile of Iclusig as the proportion of patients on the children. These include adverse effects on blood lipid lev- drug experiencing vascular occlusion events such as blood els, increased risk of heart attack and stroke, masculiniza- clots and severe narrowing of blood vessels was tion of women, shrinkage of the testicles, breast enlarge- significantly greater than the proportion reported at the ment, infertility in males, and short stature in children. -

Wednesday, February 14, 2018 4:00Pm
Wednesday, February 14, 2018 4:00pm Oklahoma Health Care Authority 4345 N. Lincoln Blvd. Oklahoma City, OK 73105 The University of Oklahoma Health Sciences Center COLLEGE OF PHARMACY PHARMACY MANAGEMENT CONSULTANTS MEMORANDUM TO: Drug Utilization Review (DUR) Board Members FROM: Bethany Holderread, Pharm.D. SUBJECT: Packet Contents for Board Meeting – February 14th, 2018 DATE: January 26, 2018 NOTE: The DUR Board will meet at 4:00 p.m. The meeting will be held at 4345 N. Lincoln Blvd. Enclosed are the following items related to the February meeting. Material is arranged in order of the agenda. Call to Order Public Comment Forum Action Item – Approval of DUR Board Meeting Minutes – Appendix A Update on Medication Coverage Authorization Unit/Chronic Medication Adherence Program Update – Appendix B Action Item – Vote to Prior Authorize Zerviate™ (Cetirizine Ophthalmic Solution) – Appendix C Action Item – Vote to Prior Authorize ArmonAir™ RespiClick® (Fluticasone Propionate), Trelegy™ Ellipta® (Fluticasone Furoate/Umeclidinium/Vilanterol), QVAR® RediHaler™ (Beclomethasone Dipropionate), AirDuo™ RespiClick® (Fluticasone Propionate/Salmeterol), and Fasenra™ (Benralizumab) and to Update Nucala® (Mepolizumab) and Xolair® (Omalizumab) Criteria – Appendix D Action Item – Vote to Prior Authorize Emflaza® (Deflazacort) – Appendix E Action Item – Vote to Prior Authorize Zilretta™ (Triamcinolone Acetonide Extended-Release Injectable Suspension) – Appendix F Action Item – Vote to Prior Authorize Varubi® IV (Rolapitant) and Cinvanti™ (Aprepitant) – Appendix G Action Item – Annual Review of Seizure Medications – Appendix H Annual Review of Osteoporosis Medications and 30-Day Notice to Prior Authorize Tymlos™ (Abaloparatide) – Appendix I Annual Review of Antiviral Medications and 30-Day Notice to Prior Authorize Prevymis™ (Letermovir Tablets and Injection) – Appendix J Annual Review of Glaucoma Medications and 30-Day Notice to Prior Authorize Rhopressa® (Netarsudil Ophthalmic Solution) and Vyzulta™ (Latanoprostene Bunod Ophthalmic Solution) – Appendix K ORI-4403 • P.O. -

Annual Report 2020 We Are a Science-Led Global Healthcare Company
Annual Report 2020 We are a science-led global healthcare company 2020 performance summary £34.1bn AER +1% £9.7bn AER +11% Group turnover CER +3% New and specialty medicines CER +12% £7.8bn AER +12% £8.9bn AER - 1% Total operating profit CER +15% Adjusted operating profit CER +2% 115.5p AER +23% 115.9p AER - 6% Total earnings per share CER +26% Adjusted earnings per share CER - 4% 9 80p 1st 2nd major pipeline Dividend in the Access to in the pharmaceutical approvals Medicine Index industry for Dow Jones Sustainability Index Contents Strategic report Our business model 01 Board roles and responsibilities 86 Financial statements of Chairman’s statement 03 Board activity and principal decisions 87 GlaxoSmithKline plc prepared CEO’s statement 04 Our purpose, values and culture 90 under UK GAAP 238 Financial performance 06 The Board’s approach to engagement 91 Our long-term priorities 09 Board performance 94 Investor information Our culture 10 Board Committee information 96 Quarterly trend 244 Key performance indicators 11 Our Board Committee reports 97 Five-year record 249 Industry trends 12 Section 172 statement 108 Product development pipeline 255 Stakeholder engagement 16 Directors’ report 109 Products, competition and Innovation 18 intellectual property 258 Remuneration report Performance 28 Principal risks and uncertainties 261 Trust 33 Chairman’s annual statement 112 Share capital and share price 276 Risk management 43 Annual report on remuneration 114 Dividends 278 Group financial review 50 2020 Remuneration policy summary 133 Financial -

Drug Pipeline Monthly Update September 2017
Drug Pipeline Monthly Update Critical updates in an ever changing environment September 2017 New drug information ● Zerviate™ (cetirizine ophthalmic solution): The U.S. Food and Drug Administration (FDA) approved Nicox Ophthalmic’s Zerviate for the twice daily treatment of ocular itching associated with allergic conjunctivitis. ● Benznidazole Tablets: The FDA granted accelerated approval to Chemo Research’s Benznidazole Tablets for the treatment of Chagas disease (American trypanosomiasis) caused by Trypanosoma cruzi in pediatric patients 2 to 12 years of age. Prior to approval, this drug was only available through the Centers for Disease Control and Prevention. The Chemo group worked with its U.S.-based pharmaceutical division Exeltis, as well as Mundo Sano and Drugs for Neglected Diseases initiative (DNDi) to complete the New Drug Application and notes that a substantial part of any revenue derived from the future sale of the neglected tropical disease priority review voucher they received will be directed towards enhancing access to treatment for Chagas patients and improving patient health in other disease areas.1 ● Zypitamag™ (pitavastatin magnesium): Zydus Pharmaceuticals received FDA approval for Zypitamag for high cholesterol. Zypitamag is an alternate salt product of Kowa Company Ltd’s Livalo® (pitastatin calcium). ● Admelog® (insulin lispro injection): The FDA granted tentative approval for Sanofi’s Admelog for the treatment of adults and children with diabetes mellitus. Tentative approval from the FDA means that Admelog met all necessary regulatory requirements for approval in the United States, pending any patent issues yet to be resolved. ● Adzenys™ ER (amphetamine): Neos Therapeutics received FDA approval of Adzenys ER, amphetamine extended-release oral suspension for the treatment of attention deficit hyperactivity disorder (ADHD) in patients aged 6 years and older. -

5 CLINICAL NEWS 16 FINANCIAL NEWS Deals, Sales & Marketing, Other Regulatory, Clinical Results, Completed Offerings, Other News Clinical Status Financial News
WEEK OF SEPTEMBER 25, 2017 1 COMPANY NEWS 5 CLINICAL NEWS 16 FINANCIAL NEWS Deals, Sales & Marketing, Other Regulatory, Clinical Results, Completed Offerings, Other News Clinical Status Financial News COMPANY NEWS line data are expected in mid-2018 from Phase III trials to treat myelodysplastic syndromes (MDS) and beta-thalassemia (see DEALS BioCentury, Aug. 8, 2011). Luspatercept is a modifiedACVR2B fusion protein that inhibits Acceleron Pharma Inc. (NASDAQ:XLRN), Cambridge, Mass. several ligands in the transforming growth factor (TGF) beta (NASDAQ:CELG), Summit, N.J. Celgene Corp. superfamily. Business: Cancer, Hematology, Cardiovascular Acceleron Pharma Inc. (NASDAQ:XLRN) gained worldwide Arcus Biosciences, Hayward, Calif. rights to develop and commercialize sotatercept to treat pulmonary Taiho Pharmaceutical Co. Ltd., Tokyo, Japan hypertension, amending a deal with partner Celgene Corp. Business: Cancer (NASDAQ:CELG). Acceleron expects to begin a Phase II trial of Cancer company Arcus Biosciences (Hayward, Calif.) granted Taiho sotatercept to treat pulmonary arterial hypertension (PAH) in 1H18. Pharmaceutical Co. Ltd. (Tokyo, Japan) an option to develop and In 2008, Acceleron and Celgene partnered to co-develop and commercialize compounds in Japan and other Asian territories commercialize Acceleron's sotatercept for all indications, with an excluding China. Arcus said the deal includes anything listed on its initial focus on cancer, including breast and ovarian cancers, and pipeline chart. cancer-related bone loss. At the time, Acceleron received $50 million Arcus will receive $35 million over the first three years of the five- up front, including a $5 million equity investment by Celgene, and year agreement and is eligible to receive up to $275 million in was eligible for up to $510 million in milestones, plus tiered royalties milestones for each molecule, plus high-single to mid-double-digit (see BioCentury, Feb. -

Group Companies
Strategic report Governance and remuneration Financial statements Investor information Other statutory disclosures continued Group companies In accordance with Section 409 of the Companies Act 2006 a full list of subsidiaries, associates, joint ventures and joint arrangements, the address of the registered office and effective percentage of equity owned, as at 31 December 2019 are disclosed below. Unless otherwise stated the share capital disclosed comprises Ordinary shares which are indirectly held by GlaxoSmithKline plc. The percentage held by class of share is stated where this is less than 100%. Unless otherwise stated, all subsidiary companies have their registered office and are tax resident in their country of incorporation. Name Security Registered address Wholly owned subsidiaries 1506369 Alberta ULC Common 3500 855-2nd Street SW, Calgary, AB, T2P 4J8, Canada Action Potential Venture Capital Limited Ordinary 980 Great West Road, Brentford, Middlesex, TW8 9GS, England Adechsa GmbH (ii) Ordinary c/o PRV Provides Treuhandgesellschaft AG, Dorfstrasse 38, Baar, 6341, Switzerland Affymax Research Institute Common Corporation Service Company, 2710 Gateway Oaks Drive, Suite 150N, Sacramento, California, 95833, United States Alenfarma – Especialidades Farmaceuticas, Limitada (ii) Ordinary Quota Rua Dr Antonio Loureiro Borges No 3, Arquiparque, Miraflores, Alges, 1495-131, Portugal Allen & Hanburys Limited (ii) Ordinary 980 Great West Road, Brentford, Middlesex, TW8 9GS, England Allen & Hanburys Pharmaceutical Nigeria Limited Ordinary 24 Abimbola Way, Ilasamaja, Isolo, Lagos, Nigeria Allen Farmaceutica, S.A. Ordinary Severo Ochoa, 2, Parque Tecnologico de Madrid, Tres Cantos, Madrid, 28760, Spain Allen Pharmazeutika Gesellschaft m.b.H. Ordinary Wagenseilgasse 3, Euro Plaza, Gebäude I, 4. Stock, Vienna, A-1120, Austria Barrier Therapeutics, Inc.