Bspublishers
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

ETHIOPIA Food Security Outlook Update November 2011
ETHIOPIA Food Security Outlook Update November 2011 Good rains likely to stabilize food security in the south The October to December Deyr rains are performing well Figure 1. Most-likely food security outcomes (October in most parts of the southern and southeastern pastoral to December 2011) and agropastoral areas, easing the shortage of pastoral resources. This, coupled with ongoing humanitarian assistance, will continue to stabilize food security among poor and very poor households in these areas. Nonetheless, about 4 million people will continue to require humanitarian assistance through the end of 2011 across the country. Prices of staple foods have generally started declining following the fresh Meher harvest, although they remain higher than the five‐year average. This will continue to constrain access to food over the coming months among the rural and urban poor who heavily depend on purchase to fulfill their minimum food requirements. For more information on FEWS NET’s Food Insecurity Severity Scale, During the January to March 2012 period, Crisis level food please see: www.fews.net/FoodInsecurityScale insecurity will extend to the dominantly Belg producing Source: FEWS NET Ethiopia and WFP zones in the northeastern highlands as well as into some marginal Meher cropping areas due to the below normal Figure 2. Most‐likely food security outcomes (January to 2011 harvests. Similarly, as the long dry season March 2012) (December to March) progresses, deterioration in food security is likely in some southern pastoral and agropastoral woredas which were severely affected by the recent drought. Updated food security outlook through March 2012 Food security in most parts of the country has stabilized as a result of improved market supply and declining prices following the Meher harvest, ongoing humanitarian assistance, and the current good Deyr/Hageya rains in the southern and southeast pastoral and agropastoral. -
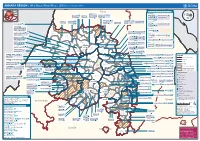
AMHARA REGION : Who Does What Where (3W) (As of 13 February 2013)
AMHARA REGION : Who Does What Where (3W) (as of 13 February 2013) Tigray Tigray Interventions/Projects at Woreda Level Afar Amhara ERCS: Lay Gayint: Beneshangul Gumu / Dire Dawa Plan Int.: Addis Ababa Hareri Save the fk Save the Save the df d/k/ CARE:f k Save the Children:f Gambela Save the Oromia Children: Children:f Children: Somali FHI: Welthungerhilfe: SNNPR j j Children:l lf/k / Oxfam GB:af ACF: ACF: Save the Save the af/k af/k Save the df Save the Save the Tach Gayint: Children:f Children: Children:fj Children:l Children: l FHI:l/k MSF Holand:f/ ! kj CARE: k Save the Children:f ! FHI:lf/k Oxfam GB: a Tselemt Save the Childrenf: j Addi Dessie Zuria: WVE: Arekay dlfk Tsegede ! Beyeda Concern:î l/ Mirab ! Concern:/ Welthungerhilfe:k Save the Children: Armacho f/k Debark Save the Children:fj Kelela: Welthungerhilfe: ! / Tach Abergele CRS: ak Save the Children:fj ! Armacho ! FHI: Save the l/k Save thef Dabat Janamora Legambo: Children:dfkj Children: ! Plan Int.:d/ j WVE: Concern: GOAL: Save the Children: dlfk Sahla k/ a / f ! ! Save the ! Lay Metema North Ziquala Children:fkj Armacho Wegera ACF: Save the Children: Tenta: ! k f Gonder ! Wag WVE: Plan Int.: / Concern: Save the dlfk Himra d k/ a WVE: ! Children: f Sekota GOAL: dlf Save the Children: Concern: Save the / ! Save: f/k Chilga ! a/ j East Children:f West ! Belesa FHI:l Save the Children:/ /k ! Gonder Belesa Dehana ! CRS: Welthungerhilfe:/ Dembia Zuria ! î Save thedf Gaz GOAL: Children: Quara ! / j CARE: WVE: Gibla ! l ! Save the Children: Welthungerhilfe: k d k/ Takusa dlfj k -

English-Full (0.5
Enhancing the Role of Forestry in Building Climate Resilient Green Economy in Ethiopia Strategy for scaling up effective forest management practices in Amhara National Regional State with particular emphasis on smallholder plantations Wubalem Tadesse Alemu Gezahegne Teshome Tesema Bitew Shibabaw Berihun Tefera Habtemariam Kassa Center for International Forestry Research Ethiopia Office Addis Ababa October 2015 Copyright © Center for International Forestry Research, 2015 Cover photo by authors FOREWORD This regional strategy document for scaling up effective forest management practices in Amhara National Regional State, with particular emphasis on smallholder plantations, was produced as one of the outputs of a project entitled “Enhancing the Role of Forestry in Ethiopia’s Climate Resilient Green Economy”, and implemented between September 2013 and August 2015. CIFOR and our ministry actively collaborated in the planning and implementation of the project, which involved over 25 senior experts drawn from Federal ministries, regional bureaus, Federal and regional research institutes, and from Wondo Genet College of Forestry and Natural Resources and other universities. The senior experts were organised into five teams, which set out to identify effective forest management practices, and enabling conditions for scaling them up, with the aim of significantly enhancing the role of forests in building a climate resilient green economy in Ethiopia. The five forest management practices studied were: the establishment and management of area exclosures; the management of plantation forests; Participatory Forest Management (PFM); agroforestry (AF); and the management of dry forests and woodlands. Each team focused on only one of the five forest management practices, and concentrated its study in one regional state. -

Flood-Based Farming for Food Security
Transition from Traditional to Modern Spate Irrigation: The Case in Kobo – Girana Valley, Amhara Region Belay Zegeye,1 Debebe Lijalem2 and Aschalew Kassie3 1MSc in Hydraulics and Hydropower Engineering, Amhara Design and Supervision Works Enterprise, Email: [email protected] 2MSc in Engineering Hydrology, Amhara Design and Supervision Works Enterprise, Email: [email protected] 3Msc in Soil Science, Amhara Design and Supervision Works Enterprise, Email: [email protected] Abstract In Ethiopia spate irrigation is on the increase due to public interest as well as farmer’s initiative. Though there are newly introduced areas, there are some spate irrigation practices that have been in use for several generations. The technology is in increasing the arid parts of the country. Kobo-Girana Valley is one of the historically practiced areas, having ample potential for spate irrigation. A potential assessment made by Amhara Design and Supervision Works Enterprise showed that an area of 51,668 ha has been identified as a potential for spate irrigation in the Kobo-Girana Valley. In order to reduce the challenges in the traditional system the newly introduced modern spate irrigation also has some limitations that require further research and design considerations. The main problem in the traditional system is frequent damage of structures and labor-intensive mismanagement of silt and water. In similar ways, the modern schemes are also underutilized due to mismanagement of the schemes and absence of proper openings for removing silt and excess water. Key words: Spate, Kobo Girana Valley, traditional, modern spate 1. Introduction Spate irrigation is a form of water management that is unique to semiarid environments, particularly where mountain catchments border lowlands (Steenbergen et al. -

Ethiopia: Amhara Region Administrative Map (As of 05 Jan 2015)
Ethiopia: Amhara region administrative map (as of 05 Jan 2015) ! ! ! ! ! ! ! ! ! ! Abrha jara ! Tselemt !Adi Arikay Town ! Addi Arekay ! Zarima Town !Kerakr ! ! T!IGRAY Tsegede ! ! Mirab Armacho Beyeda ! Debark ! Debarq Town ! Dil Yibza Town ! ! Weken Town Abergele Tach Armacho ! Sanja Town Mekane Berhan Town ! Dabat DabatTown ! Metema Town ! Janamora ! Masero Denb Town ! Sahla ! Kokit Town Gedebge Town SUDAN ! ! Wegera ! Genda Wuha Town Ziquala ! Amba Giorges Town Tsitsika Town ! ! ! ! Metema Lay ArmachoTikil Dingay Town ! Wag Himra North Gonder ! Sekota Sekota ! Shinfa Tomn Negade Bahr ! ! Gondar Chilga Aukel Ketema ! ! Ayimba Town East Belesa Seraba ! Hamusit ! ! West Belesa ! ! ARIBAYA TOWN Gonder Zuria ! Koladiba Town AMED WERK TOWN ! Dehana ! Dagoma ! Dembia Maksegnit ! Gwehala ! ! Chuahit Town ! ! ! Salya Town Gaz Gibla ! Infranz Gorgora Town ! ! Quara Gelegu Town Takusa Dalga Town ! ! Ebenat Kobo Town Adis Zemen Town Bugna ! ! ! Ambo Meda TownEbinat ! ! Yafiga Town Kobo ! Gidan Libo Kemkem ! Esey Debr Lake Tana Lalibela Town Gomenge ! Lasta ! Muja Town Robit ! ! ! Dengel Ber Gobye Town Shahura ! ! ! Wereta Town Kulmesk Town Alfa ! Amedber Town ! ! KUNIZILA TOWN ! Debre Tabor North Wollo ! Hara Town Fogera Lay Gayint Weldiya ! Farta ! Gasay! Town Meket ! Hamusit Ketrma ! ! Filahit Town Guba Lafto ! AFAR South Gonder Sal!i Town Nefas mewicha Town ! ! Fendiqa Town Zege Town Anibesema Jawi ! ! ! MersaTown Semen Achefer ! Arib Gebeya YISMALA TOWN ! Este Town Arb Gegeya Town Kon Town ! ! ! ! Wegel tena Town Habru ! Fendka Town Dera -

AMHARA Demography and Health
1 AMHARA Demography and Health Aynalem Adugna January 1, 2021 www.EthioDemographyAndHealth.Org 2 Amhara Suggested citation: Amhara: Demography and Health Aynalem Adugna January 1, 20201 www.EthioDemographyAndHealth.Org Landforms, Climate and Economy Located in northwestern Ethiopia the Amhara Region between 9°20' and 14°20' North latitude and 36° 20' and 40° 20' East longitude the Amhara Region has an estimated land area of about 170000 square kilometers . The region borders Tigray in the North, Afar in the East, Oromiya in the South, Benishangul-Gumiz in the Southwest and the country of Sudan to the west [1]. Amhara is divided into 11 zones, and 140 Weredas (see map at the bottom of this page). There are about 3429 kebeles (the smallest administrative units) [1]. "Decision-making power has recently been decentralized to Weredas and thus the Weredas are responsible for all development activities in their areas." The 11 administrative zones are: North Gonder, South Gonder, West Gojjam, East Gojjam, Awie, Wag Hemra, North Wollo, South Wollo, Oromia, North Shewa and Bahir Dar City special zone. [1] The historic Amhara Region contains much of the highland plateaus above 1500 meters with rugged formations, gorges and valleys, and millions of settlements for Amhara villages surrounded by subsistence farms and grazing fields. In this Region are located, the world- renowned Nile River and its source, Lake Tana, as well as historic sites including Gonder, and Lalibela. "Interspersed on the landscape are higher mountain ranges and cratered cones, the highest of which, at 4,620 meters, is Ras Dashen Terara northeast of Gonder. -

Periodic Monitoring Report Working 2016 Humanitarian Requirements Document – Ethiopia Group
DRMTechnical Periodic Monitoring Report Working 2016 Humanitarian Requirements Document – Ethiopia Group Covering 1 Jan to 31 Dec 2016 Prepared by Clusters and NDRMC Introduction The El Niño global climactic event significantly affected the 2015 meher/summer rains on the heels of failed belg/ spring rains in 2015, driving food insecurity, malnutrition and serious water shortages in many parts of the country. The Government and humanitarian partners issued a joint 2016 Humanitarian Requirements Document (HRD) in December 2015 requesting US$1.4 billion to assist 10.2 million people with food, health and nutrition, water, agriculture, shelter and non-food items, protection and emergency education responses. Following the delay and erratic performance of the belg/spring rains in 2016, a Prioritization Statement was issued in May 2016 with updated humanitarian requirements in nutrition (MAM), agriculture, shelter and non-food items and education.The Mid-Year Review of the HRD identified 9.7 million beneficiaries and updated the funding requirements to $1.2 billion. The 2016 HRD is 69 per cent funded, with contributions of $1.08 billion from international donors and the Government of Ethiopia (including carry-over resources from 2015). Under the leadership of the Government of Ethiopia delivery of life-saving and life- sustaining humanitarian assistance continues across the sectors. However, effective humanitarian response was challenged by shortage of resources, limited logistical capacities and associated delays, and weak real-time information management. This Periodic Monitoring Report (PMR) provides a summary of the cluster financial inputs against outputs and achievements against cluster objectives using secured funding since the launch of the 2016 HRD. -

Determinants of Vegetables Market Supply in Case of Habru District, North Wollo Zone, Ethiopia
IJSAR, 7(4), 2020; 01-12 International Journal of Sciences & Applied Research www.ijsar.in Determinants of vegetables market supply in case of Habru district, North Wollo Zone, Ethiopia Ebrahim Endris1*, Jema Haji2, Bosena Tegegne3 1Department of Agricultural Economics, Woldia University, Woldia, Ethiopia. 2School of Agricultural Economics and Agribusiness, Haramaya University, Haramaya, Ethiopia. 3School of Agricultural Economics and Agribusiness, Haramaya University, Haramaya, Ethiopia. Corresponding author: *Ebrahim Endris, Department of Agricultural Economics, Woldia University, Woldia, Ethiopia. _____________________________________________________________________________________________ Abstract The development of vegetable subsector in Habru district faces various marketing problems to supply their produce to the market. Therefore, this study was aimed to identify the determinants of vegetables market supply in Habru district. Data for the study were collected from primary and secondary sources. Primary data were collected from a randomly selected 147 vegetable producer households. Descriptive statistics and multiple regression econometric methods were used for identifying determinants of vegetable market supply. The test for assumptions of the classical linear regression model showed endogeneity in market supply analysis and hetero skedasticity problem in tomato supply analysis. Two-stage least squares result showed that quantity produced, farming experience and extension contact significantly and positively affected the quantity -
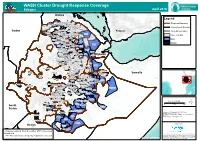
WASH Cluster Drought Response Coverage
WASH Cluster Drought Response Coverage Ethiopia April 2016 Eritrea Legend Ahferom Laelay Adiyabo Mereb Leke Erob Gulomekeda Regional Boundary Tahtay Adiyabo Medebay Zana Adwa Ganta Afeshum Dalul Kafta Humera Saesie Tsaedaemba International Boudary Hawzen Koneba Berahile Naeder Adet Atsbi Wenberta Sudan Tselemti Kelete Awelallo Yemen Woreda Boundary Tsegede Addi Arekay Tselemti Enderta Tselemt Saharti Samre Ab Ala Afdera Tsegede Tanqua Abergele Debark Erebti GoE + NGOs Abergele Hintalo Wejirat Janamora Alaje Bidu Sahla Wegera Ziquala GoE Sekota Yalo Teru East Belesa Ofla Kurri West Belesa Dehana Alamata NGOs Gaz Gibla Elidar Ebenat Bugna Kobo Gulina Awra Lasta Ewa Lay Gayint Meket Guba Lafto Delanta Dubti Tach Gayint Wadla Habru Chifra Aysaita Dawunt Worebabu Simada Ambasel Adaa'r Mile Sayint Mekdela Bati Afambo Djibouti Tenta Kalu Mehal Sayint Argoba Enbise Sar Midir Legambo Dewa Cheffa Dewe Enarj Enawga Debresina Legehida Dewa Harewa Were Ilu Dalfagi Kelela Artuma Fursi Gewane Jama Erer Shebel Bereta Menz Gera Midir Hadelela Eferatana Gidem Shinile Mimo Weremo Afdem Merahbete Jille Timuga Kewet Jarte Jardega Moretna Jiru Amibara Ensaro Simurobi Gele'alo Miesso Argoba Special Chinaksen Kembibit Ankober Jarso Jarso Ambo Zuria Assagirt Meta Somalia Kembibit Gursum Jijiga Sasiga Awash Fentale Mieso Doba Gursum Chiro Zuria Babile Diga Berehet Goba Koricha Toke Kutaye Minjar Shenkora Gemechis Habro Bedeno Dendi Malka Balo Girawa Boset Anchar Babile Kuni Midega Tola Adama Daro Lebu Boke Golo Oda Jeju Itang Chora Sodo Dodota Halu (Huka) Muhur -

The Multifaceted Commodification Processes and Transformations of Pastoralists in Lowland Ethiopia
The Multifaceted Commodification Processes and Transformations of Pastoralists in Lowland Ethiopia by Olivia Pearson A Cumulative Dissertation in Geography Faculty of Applied Computer Sciences University of Augsburg Germany June 2017 First Referee (Supervisor): Prof. Dr. Matthias Schmidt Second Referee: apl. Prof. Dr. Karin Thieme Date of oral examination: 05/10/2017 i The Multifaceted Commodification Processes and Transformations of Pastoralists in Lowland Ethiopia Vorgelegt von Olivia Pearson Kumulative Dissertation Fachgebiet: Geographie Fakultät für Angewandte Informatik der Universität Augsburg Deutschland Juni, 2017 Erstgutachter (Betreuer): Prof. Dr. Matthias Schmidt Zweitgutachterin: apl. Prof. Dr. Karin Thieme Datum der mündlichen Prüfung: 05.10.2017 Page | ii TABLE OF CONTENTS Table of Contents .............................................................................................................. i List of Figures .......................................................................................................................... iii List of Tables ........................................................................................................................... iii Acronyms and Glossary ........................................................................................................... iv Acknowledgements .................................................................................................................. v Executive Summary ............................................................................................................... -

Gender Responsive Pedagogy: Practices, Challenges & Opportunities
Gender Responsive Pedagogy: Practices, Challenges & Opportunities - A Case of Secondary Schools of North Wollo Zone, Ethiopia Mollaw Abraha1*, Asrat Dagnew2 and Amera Seifu2 1Education and Behavioral Sciences, Woldia University, Post Box No: 400, Ethiopia 2Education and Behavioral Sciences, Bahir Dar University, Post Box No: 1876, Bahir Dar, Ethiopia Abstract The purpose of the current study was to examine the general secondary school (GSS) science teachers’ gender responsive pedagogy (GRP) implementation status. To do so, descriptive survey research design was employed. Teachers, department heads and school principals were taken as respondents of the study comprehensively. And students were also participants of the study conveniently. Questionnaire, interview, and focus group discussion (FGD) were considered as the principal data collection tools. The collected data was analyzed both qualitatively and quantitatively: the data collected through questionnaire was analyzed via mean, std., one sample t-test. And the data gathered by open ended items, interview, and FGD as well was analyzed via words, phrases, statements and narration. The analyzed data noted that GSS science teachers of the North Wollo Zone had facilitated GRP fairly. Thus, they found as effective in relation to language usage, classroom setups, classroom interaction, and addressing sexual harassment. To do so, availability of qualified school supervisors and principals, and realization of new education training policy (NETP) were considered as possible opportunities. However, teachers as well ineffective to prepare gender responsive (GR) lesson plan, to prepare and use GR instructional materials and to have GR management of sexual maturation. This vanity of teachers was sourced from ranges of challenges: economic, culture, school and teacher related. -

Loans and Training Help Raise Ethiopian Women from Poverty
TM Loans and Training Help Raise Project Profile Organization: REPORT Ethiopian Women From Poverty Women Support Organization (WSO) Grant amount: $22,618 Geneva Global ID: 1-Z7E7-1106 Project duration: DELTA Members celebrate 18 months the first anniversary of the Edeget self- help group in Expected Performance Merssa, Ethiopia, in March 2004. DELTA SCORE Measures relative grant effectiveness 4 5.3 6.6 RISK-ADJUSTED RANGE GRANT PROFILE Reflects aggregate project risk Overview CONSERVATIVE AGGRESSIVE In rural Ethiopian regions stricken by drought and food shortages, women who farm to feed families struggle with a lack of access to affordable credit and skills training. WSO Please refer to the FAQ at the end of this document for an creates self-help groups that provide members with small loans, training and support. explanation of the Delta Score and Grant Profile. Hundreds of women will gain capital for business growth, learn entrepreneurial skills Project Location and increase farm income, raising the quality of life for hundreds more family members. Region Expected Life Change Results An investment of $22,618 expands WSO’s program providing microloans and training to women and community leaders in two additional rural areas. Expected Life Change: 300 women gain entrepreneurial skills and loans Country 1,800 women and family members benefit from increased family income 100 community leaders gain understanding of women’s rights and HIV prevention 100 women have their rights protected 1,800 people impacted – some in multiple ways – at a cost per life changed of $12.57 What We Like About This Project ETHIOPIA WSO helps combat poverty by strategically empowering rural women with practical skills and opportunities for credit, resulting in increased household food supplies.